Abstract
Emerging evidence suggests that our memories for recent events depend on a dynamic interplay between multiple cortical brain regions, although previous research has also emphasized a primary role for the hippocampus in episodic memory. One challenge in determining the relative importance of interactions between multiple brain regions versus a specific brain region is a lack of analytic approaches to address this issue. Participants underwent neuroimaging while retrieving the spatial and temporal details of a recently experienced virtual reality environment; we then employed graph theory to analyze functional connectivity patterns across multiple lobes. Dense, large-scale increases in connectivity during successful memory retrieval typified network topology, with individual participant performance correlating positively with overall network density. Within this dense network, the hippocampus, prefrontal cortex, precuneus and visual cortex served as “hubs” of high connectivity. Spatial and temporal retrieval were characterized by distinct but overlapping “subnetworks” with higher connectivity within posterior and anterior brain areas, respectively. Together, these findings provide new insight into the neural basis of episodic memory, suggesting that the interactions of multiple hubs characterize successful memory retrieval. Furthermore, distinct subnetworks represent components of spatial versus temporal retrieval, with the hippocampus acting as a hub integrating information between these two subnetworks.
Similar content being viewed by others
Introduction
Spatial and temporal details constitute critical components of our memories for recently experienced events, termed episodic memories. Both knowledge about where we were and approximately when things happened during our day are important cues for remembering what happened to us at an earlier event. Current literature strongly supports the involvement of the hippocampus generally in storage and retrieval of episodic memories1, specifically in coding both spatial and temporal elements of recent events2,3,4,5,6. Yet, past studies have also implicated regions outside of the medial temporal lobes in episodic memory, such as the prefrontal cortex and the relative importance of this and other cortical areas to episodic memory remains under debate7,8. In support of the participation of neocortical regions to episodic memory, lesion studies implicate the prefrontal cortex and posterior parietal regions in episodic memory functions9,10. In particular, lesions to prefrontal cortex have also been linked with more specific deficits in temporal order processing11, while lesions to parietal/retrosplenial areas are linked to more specific deficits in spatial processing12. Thus, the relative contributions of cortical areas outside of the hippocampus to episodic memory and the exact role of these areas – including the hippocampus – in coordinating spatiotemporal information from disparate neocortical areas, remains unresolved.
Examining successful compared to unsuccessful retrieval of spatiotemporal details, we tested three hypotheses regarding large-scale brain networks using functional magnetic resonance imaging (fMRI). Correct retrieval might involve greater connectivity to a primary region (e.g., the hippocampus), suggesting it depends principally on a single brain area. Alternatively, correct memory retrieval could involve greater increases overall in functional connectivity compared to incorrect retrieval, with little contribution from specific regions. Finally, correct memory retrieval might involve some combination of the two (overall increase in connectivity and overall greater connectivity to multiple areas). This final hypothesis is most consistent with evidence from previous research because it incorporates and emphasizes the roles of additional areas (such as prefrontal and parietal cortices) in episodic memory retrieval. Our experimental paradigm involved participants retrieving both spatial and temporal order information independently from a recently acquired experience, thus enabling us to contrast these components of episodic memory. To further elucidate these neural correlates, based on past literature2,13,14, we propose that retrieval of these separate contextual details could involve distinct subnetworks within a larger episodic memory retrieval network, involving a limited but distinct set of overlapping hubs (i.e., the hippocampus). Or, spatial and temporal order retrieval might involve largely overlapping networks, in which case we would not find differences when contrasting spatial versus temporal order retrieval. To address these competing hypotheses, we chose seed brain regions from 13 bilateral brain areas suggested in past work to be involved in some form in episodic memory literature15,16 but also those related to other processes, such as attention and working memory. These areas formed the basis for a graph theory analysis in which we directly compared both correct and incorrect memory retrieval and spatial and temporal retrieval more generally.
Results
Participants first navigated a virtual reality environment in which they learned both the spatial layout of the city and the temporal order of deliveries of a passenger to different stores (Fig. 1A). Following encoding, we probed participants' contextual memory for their virtual reality experience (Fig. 1B) while undergoing fMRI. Questions consisted of a reference store followed by two probe stores; participants indicated which of the two probe stores was closer in either spatial or temporal distance from the reference store. Behavioral accuracy was 64.4 ± 9.7% for spatial and 61.5 ± 9.7% (mean ± s.d.) for temporal questions. Importantly, we found no difference in performance between conditions (paired t test, t(15) = 0.954, p = 0.355; for a complete description of the behavioral design and implementation, see Ekstrom et al., 2011). Analyzing the fMRI data, we first focused on two fundamental contrasts using functional connectivity and graph theory: 1) correct versus incorrect retrieval (i.e., trials on which the participants correctly judged the spatial or temporal distance compared to incorrect trials) and 2) spatial compared to temporal retrieval trials.
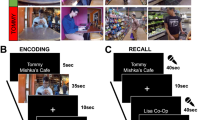
Description of Experimental Design.
(A) Participants (n = 16) navigated a virtual city where they picked up a passenger (depicted in the left panel) and delivered the person to a series of stores; they encoded the spatial layout of the city and the temporal order of deliveries. The middle panel provides an example of a store the participant visited. The right panel shows an aerial view of the virtual environment with the stores distributed unevenly around a circle. (B) After encoding, participants underwent functional imaging while performing the spatiotemporal retrieval task shown here. Participants were first presented with a single reference store and asked to indicate whether they saw this store before. After responding, they were subsequently presented with the spatiotemporal contextual retrieval question. Participants were shown two additional store pictures and asked to make a distance judgment regarding the spatial or temporal proximity to the reference store.
Successful versus unsuccessful memory retrieval
To create our retrieval networks, we used a beta time series analysis17, which assumes two regions are coupled during a specific condition of a task if the activity of both is significantly correlated across trials. In other words, two regions are functionally connected if their responses fluctuate similarly under the same condition. To characterize differential connectivity between conditions (correct versus incorrect contextual retrieval), we took the set difference between two networks, i.e., connections that were exclusive to either correct or incorrect contextual retrieval (labeled here as correct – incorrect and incorrect – correct, see Methods for analytic and statistical details). When participants accurately judged the spatial or temporal distance of probe questions compared to incorrect trials, we found a significant overall increase in large-scale network connectivity, indexed by the number of connections (also termed edges) between our regions of interest (also termed nodes) (χ2 (1) = 63.75, p < 0.0001, Fig. 2A,B,C). This result suggests that the increase in coordinated activity of multiple distributed regions underlies successful retrieval of spatiotemporal contextual details.
Increases in Network Connectivity during Successful Retrieval of Spatiotemporal Details.
(A and B) Each ROI (colored circle) is plotted with significant connections (pink lines) between the nodes; network during correct context responses shown in A and during incorrect context responses in B. Both size and color indicate node degree; a larger radius and warmer colors represent a higher node degree, while a smaller radius and cooler colors represent a lower node degree. The ROIs are spatially distributed according to their x and y coordinates in standardized brain space but have been collapsed in the z coordinate direction. The right panel displays node hierarchy where anterior nodes (red) and posterior nodes (blue) are plotted in ascending order of node degree, with the highest node degree level at the top; nodes belonging to the same horizontal level have the same node degree. The abbreviations for the ROIs are as follows: SFG (Superior Frontal Gyrus); MFG (Middle Frontal Gyrus); IFOr (Inferior Frontal Gyrus, Orbitalis); IFTr (Inferior Frontal Gyrus, Triangularis); IFOp (Inferior Frontal Gyrus, Opercularis); PrCG (Precentral Gyrus); aHPC (Anterior Hippocampus); pHPC (Posterior Hippocampus); pPHG (Posterior Parahippocampal Gyrus); IPL (Inferior Parietal Lobule); SPL (Superior Parietal Lobule); PCN (Precuneus); Calc (Calcarine sulcus). (C) Left: The adjacency matrix (right of diagonal: correct – incorrect, left of diagonal: incorrect – correct) depicts the data to optimize viewing the intra-lobular connections. The colored boxes indicate intra-lobular connections in the frontal lobe and temporal lobe (blue, green, respectively). For successful retrieval, numerous connections exist within the MTL but many are found throughout the neocortex, compared to low levels of functional connectivity throughout the brain during unsuccessful retrieval. Right: Comparison of the total number of edges (significant functional connections) between conditions, with the correct – incorrect network containing significantly more edges. *p < 0.0001. (D) Bar graphs showing betweenness centrality for each ROI (collapsed across hemispheres). Asterisks indicate pcorrected < 0.05 significant differences using a chi-squared test on node connectivity between conditions (i.e. correct – incorrect and incorrect – correct).
Correct retrieval was not only indexed by the density of network connections but also by the precise organization of its edges to particular nodes. Specifically, we investigated regional importance using a combination of two metrics: node degree and betweenness centrality. Node degree captures the local connectivity of a node, while betweenness centrality describes more global aspects of the network by tracking the tendency for the connections from other nodes to travel through a given node. Previous studies have found these two graph theory metrics to be reliable predictors of functional hubs18,19,20. We found that several areas within the medial temporal lobes (MTL), frontal and parietal lobes and the visual cortex, exhibited significantly greater levels of betweenness centrality than other nodes in the network for correct compared to incorrect retrieval trials. Figure 2D shows graphs for brain hubs displaying high betweenness centrality, with chi-squared tests indicating significant differences between the two conditions. Tests were Bonferroni corrected at p < 0.05 to account for multiple comparisons between regions (all pcorrected < 0.05; Calcarine sulcus (Calc): χ2 (1) = 93.86; anterior hippocampus (aHPC): χ2 (1) = 45.92; posterior hippocampus (pHPC): χ2 (1) = 24.66; inferior frontal gyrus, pars opercularis (IFOp): χ2 (1) = 15.38; inferior frontal gyrus, pars triangularis (IFTr): χ2 (1) = 11.00). Two other hubs, precuneus and parahippocampal gyrus, showed higher betweenness centrality for correct – incorrect but at an uncorrected threshold of p < 0.05. Thus, our analyses supported the idea that correct contextual retrieval involved greater overall connectivity across multiple cortical areas, but that this connectivity was most marked at specific brain hubs, supporting our third hypothesis.
To rank the relative importance of each hub to correct spatiotemporal retrieval, we organized each node according to its total degree (i.e., total number of connections, see Methods; for a comparison of betweenness centrality and node degree, see Fig. S3). Consistent with our earlier analysis of betweenness centrality, numerous different edges were involved in correct spatiotemporal retrieval, with hippocampus, visual cortex and inferior frontal gyrus at the top of the hierarchy (Fig. 2A, right). Overall, the total connectivity in the MTL (aHPC, pHPC and pPHG) was greater than that in any other brain region (MTL: 51; next highest, visual cortex: 31, χ2 (1) = 4.88, p = 0.027), suggesting a prominent role of this area in successful retrieval of spatiotemporal contextual details (Fig. 2A). Interestingly, while successful retrieval resulted in numerous hubs and many more functional connections than incorrect retrieval, one hub did characterize the incorrect memory network: inferior frontal gyrus, pars orbitalis (IFOr). As shown in the node hierarchy in Figure 2B and in the bar graph in Figure 2D, this region of prefrontal cortex had increased participation during incorrect rather than correct retrieval (χ2 (1) = 20.02, pcorrected < 0.05). Past research has implicated the inferior frontal gyrus in suppression of prepotent responses9 and it is intriguing to consider that heightened influences of this subarea during retrieval could indicate inhibition of other circuits relevant to correct retrieval.
Network density and behavioral performance
Next, we addressed whether 1) overall network connectivity, or density, was predictive of behavioral performance and 2) if so, did specific hubs contribute greater variance than others in terms of explaining this effect. Critically, demonstrating a link between memory performance and overall network connectivity would provide strong evidence for the importance of increases in connectivity in individual participants to memory performance. We first employed simple linear regression to test if the density of individual networks could significantly predict behavioral accuracy. We did this by regressing each participant's performance against the total percent connectivity of the individual network (see Methods). We found a significant linear relationship between participant performance and the overall connectivity within their network, which is depicted in the scatterplot in Figure 3. These results indicate that the density of connections explained 33% of the variance (R2 = 0.33, F(1,15) = 6.90, p = 0.020, β = 0.22) in participant performance. This relationship was not driven by outliers as it was still significant using robust regression (p = 0.033).
Individual Patterns of Network Connectivity Predict Memory Performance.
The scatter plot shows a significant linear relationship between total connectivity and individual participant behavioral performance (p < 0.05). To determine which ROIs were driving this correlation, each node's contribution to overall network density was regressed against individual performance using a multiple linear regression. The initial model in the stepwise regression consisted of the nodes labeled with asterisks and a final more parsimonious model resulted in the nodes highlighted in red (MFG, HPC, PCN and IFG). The beta coefficients from the linear regression and stepwise regression are presented on a line next to the independent variables. For example, the network density resulted in a coefficient of 0.20 for the linear regression.
We subsequently considered how each individual node contributed to this significant linear relationship using a multivariate stepwise regression approach. Based on hubs that first showed a significant simple regression with performance, a stepwise regression using these brain areas (indicated with asterisks in Fig. 3) resulted in a final more parsimonious model with MFG, precuneus, hippocampus and IFOr (shown in red) contributing significantly to the model (β = 4.05, 6.83, 6.76, 5.08, −8.83, p = 0.0001, R2 = 0.90). These findings argue that while overall network connectivity was a significant predictor of memory performance, specific hubs drove this effect most strongly. Consistent with our earlier analysis, this included connectivity to the MFG, hippocampus, precuneus (significant for uncorrected values) and IFOr as significant predictors of performance. Coefficients for MFG, hippocampus and precuneus were positive, indicating a significant positive linear relationship. IFOr, in contrast, displayed a negative coefficient, indicating connectivity at this particular node was a negative predictor of performance. These findings largely mirror our earlier results, suggesting the importance of hippocampus, prefrontal cortex and parts of parietal cortex to successful memory retrieval. They also provide an important extension by showing a direct relationship between connectivity at specific brain hubs and individual participant memory performance.
While our results exhibited a clear relationship between node connectivity and behavior, it is reasonable to speculate that the average level of activation of each node could also contribute to, or even confound, this relationship. For example, nodes that have high levels of activation could potentially influence the correlation of the BOLD parameter estimates, thus potentially explaining their significant functional interactions in the network. To address this idea, we correlated node degree and average parameter estimates (the average of parameter estimates across participants for each ROI) and found no significant correlation (ρ = −0.01, p = 0.9614). In addition, adding the average parameter estimate for each node to the stepwise regression between node degree and behavioral performance contributed no significant variance to the overall model. These analyses suggest that univariate effects alone are unlikely to be driving our results.
Differential spatial and temporal connectivity
Comparing networks during spatial and temporal order retrieval generally, we found striking differences in their topology, as revealed by the two-dimensional anatomical plots in Figure 4A,B. During spatial retrieval, a greater number of edges were concentrated in posterior areas of the brain while during temporal retrieval, connectivity was higher in anterior parts of the brain (Fisher's exact test, p < 0.0001, Fig. 4C, middle). This was also evident from the adjacency matrices (Fig. 4C, left), where the blue and green boxes emphasize fronto-temporal connections and the red box shows connections within the entire posterior portion of the brain.
Differential Spatial and Temporal Network Connectivity.
Panels A, B and C are arranged similarly to Fig. 2 but show the spatial – temporal and temporal – spatial retrieval networks. (A, B) Connectivity plots, using standardized brain space (left plot) or hierarchical arrangement based on node degree (right plot), for spatial and temporal networks. (C) Left: Adjacency matrix where right of diagonal are significant nodes during spatial retrieval and left of diagonal are significant nodes during temporal order retrieval. Middle: Bar graphs comparing the number of edges for anterior versus posterior brain regions for the two conditions. Anterior brain regions showed higher connectivity during temporal retrieval while posterior brain regions showed higher connectivity during spatial retrieval. Right: Bar graph showing no difference in the total number of edges present in each network. (D and E) The MTL (aHPC, pHPC, pPHG) regions displayed high betweenness centrality values but no differences between condition. The SPL and calcarine ROIs showed significantly greater betweenness centrality for the spatial – temporal network, while IFG and MFG showed greater betweenness centrality in the temporal – spatial network. *pcorrected < 0.05.
As shown in Figure 4D, betweenness centrality indicated that the SPL and visual cortex had high betweenness centrality for the difference between spatial and temporal (spatial – temporal, see Methods) compared to the temporal – spatial retrieval networks (all pcorrected < 0.05, SPL: χ2 (1) = 15.41 and primary visual cortex: χ2 (1) = 20.15). Conversely, MFG and IFG nodes, located in the anterior portion of the brain, had higher betweenness centrality for temporal – spatial (all pcorrected < 0.05, MFG: χ2 (1) = 9.90, IFTr: χ2 (1) = 35.90, IFOp: χ2 (1) = 20.43, Fig. 4E). However, the MTL nodes showed levels of connectivity significantly greater than zero for both spatial and temporal order retrieval, indicating significant levels of connectivity during both forms of retrieval. Importantly, though, they exhibited no significant differences in their levels of connectivity for spatial versus temporal (all pcorrected > 0.05, aHPC: χ2 (1) = 4.30, pHPC: χ2 (1) = 4.14, pPHG: χ2 (1) = 0.11). Additionally, the node hierarchies in the right panels of Figure 4A,B emphasize a similar level of connectivity and betweenness centrality for the different conditions. Contrary to our successful memory retrieval analysis, spatial and temporal retrieval networks exhibited similar density; a chi-squared test confirmed that the number of connections is balanced between the spatial and the temporal conditions (χ2 (1) = 1.38 p = 0.24, Fig. 4C, right), arguing that differences in regional connectivity could not be accounted for by lower degrees of connectivity in one condition. Again, differences in regional activation did not account for the pattern of node connectivity as the two were uncorrelated (spatial – temporal: ρ = −0.17, p = 0.4120; temporal – spatial: ρ = −0.09, p = 0.6740). Overall, these findings support the idea that spatial and temporal retrieval contribute somewhat independently to the correct retrieval effect we reported above, acting as subnetworks that overlap within the larger network involved in episodic memory retrieval.
Discussion
The present study aimed to test several hypotheses regarding the patterns of functional connectivity underlying retrieval of spatiotemporal contextual details. By leveraging a new approach, whole brain functional connectivity with graph theory, we were able to better understand the contributions of different brain regions to episodic memory retrieval. Under the different conditions we examined (such as correct versus incorrect or spatial versus temporal trials), particular networks emerged as important to episodic memory retrieval. Specifically, successful retrieval resulted in greater overall connectivity combined with greater connectivity to specific hubs, including the hippocampus. Past fMRI studies employing univariate analyses have also focused on a primary role for the hippocampus in episodic memory5,6,21,22,23,24,25,26, although studies have also shown activation in the parahippocampal cortex6,27, prefrontal cortex8,28,29,30,31,32, precuneus31,33, visual cortex31, posterior parietal cortex/angular gyrus34,35 and retrosplenial cortex36,37. These findings have not been unified across studies, however and univariate methods alone cannot indicate whether and to what extent these disparate areas interact38. Thus, our results provide a potentially new perspective on the neural basis of episodic memory, emphasizing the importance of interactions across multiple brain regions rather than the computations of a single primary brain region (such as the hippocampus).
Our findings comparing correct versus incorrect retrieval are consistent with several theoretical models of episodic memory39,40,41 which emphasize the importance of hippocampal-neocortical interactions. At the same time, our findings provide novel evidence that the hippocampus and MTL more generally, indeed serves as a central “hub” for all forms of memory representation compared to other cortical regions. This is congruous with our previous findings using this same paradigm showing significant levels of activation in the hippocampus during both spatial and temporal order retrieval6 and other studies emphasizing the importance of the hippocampus to both spatial and temporal order retrieval2,3,4,5. The presence of the hippocampus as a primary hub in our study also provides novel insight into why the hippocampus frequently appears as a primary brain area in episodic memory.
Our findings also bolster an important perspective in the memory literature: other brain hubs, outside of the MTL, contribute significantly to episodic memory. Past lesion work supports the idea that brain damage outside of the hippocampus, including prefrontal cortex15 and parietal cortex10, impairs episodic memory, reinforcing the idea that this important function involves brain hubs across multiple lobes. Thus, while past work has also suggested the importance of extra-hippocampal brain areas to memory (for reviews, see16,42), our results suggest a reason why: prefrontal cortex, precuneus and visual cortex are densely connected during successful memory retrieval. Our results further suggest one reason why hippocampal damage, in particular, may be devastating to episodic memory. While prefrontal cortex showed greater connectivity during temporal order compared to spatial layout retrieval and parietal cortex showed greater connectivity during spatial layout retrieval compared to temporal order retrieval, only the hippocampus (and other parts of the MTL) acted as a hub during both spatial and temporal order processing. The importance of the hippocampus to spatiotemporal memory is consistent with numerous studies using human neuroimaging6,43, intracranial EEG recordings14, human lesion2,4, rat electrophysiological studies44 and rat lesion studies45 suggesting its role in both spatial and temporal components of episodic memory. Thus, one possible interpretation of our results demonstrating important roles for the prefrontal cortex, precuneus and visual cortex in episodic memory retrieval is that these areas likely facilitate and augment memory trace retrieval46,47. One proposal, consistent with past work, is that prefrontal cortex and parietal cortex may be playing more auxiliary roles in memory, such as in executive or attentional processes37,42, with visual cortex supporting the visually-rich details that often accompany memory retrieval48. These more auxiliary roles are supported by the comparatively lower levels of connectivity they showed in contrast to the hippocampus in our study. By employing graph theory and functional connectivity in combination, future studies explicitly manipulating these variables, or meta-analyses of past studies, will be better able to address these possible specific roles.
Our results converge with previous findings from our lab by Watrous and colleagues38 who used a similar behavioral paradigm with intracranial EEG in human patients to show increased global connectivity across the frontal, medial temporal and parietal lobes, with the MTL acting as a hub, during successful spatiotemporal retrieval. Previous findings have suggested similarities between low-frequency band oscillatory coherence and functional connectivity measured using fMRI49 and corresponding results from our two studies (global increases in functional interactions for correct versus incorrect retrieval) are also consistent with the idea that low-frequency oscillatory coherence and function connectivity, measured via BOLD time series correlations, may carry similar types of information. Complementary to the current results, Watrous and colleagues found that spatial and temporal retrieval arose from common anatomical but frequency-specific subnetworks, with somewhat limited regional specificity distinguishing between spatial and temporal order retrieval. While iEEG affords high spectrotemporal resolution compared to fMRI, fMRI provides potentially greater anatomical precision with greater ease of sampling a large number of brain hubs, including the hippocampus, which we did not sample in the Watrous et al. study. The two studies therefore highlight complementary aspects of retrieval, the spectrotemporal and functional/anatomical substrates of memory retrieval. Importantly, our current findings thus provide several new important advances, implicating 1) the hippocampus as a hub in all aspects of spatiotemporal memory processing and retrieval 2) more comprehensive anatomical differences for spatial versus temporal retrieval, particularly anterior versus posterior differences, than previously observed 3) unique roles for the IFOr in incorrect versus correct retrieval and 4) greater levels of functional connectivity in individual participants as important to better memory performance.
In conclusion, by employing a novel combination of fMRI, functional connectivity and a graph theory approach, our study provides a new perspective on how the human brain processes episodic memories. Our data provide novel support for models that emphasize global network interactions, rather than regionally mediated activity alone, as central to how we recover spatial and temporal memories associated with recent experiences. Our results nonetheless emphasize the importance of the hippocampus in particular as a convergence hub in this process, consistent with decades of research demonstrating its importance to episodic memory. Our results thus suggest that while prefrontal and parietal areas communicate with the hippocampus during spatiotemporal memory retrieval, the hippocampus acts as a critical convergence “hub” during successful context retrieval.
Methods
Participants
Sixteen (13 female) healthy, right-handed participants participated in this study (mean age: 21.5, range: 20–28) and were compensated for their involvement. All were recruited from the University of California, Davis and the surrounding communities and gave written informed consent, which was approved by the institutional review board at the University of California, Davis. Experiments and procedures were conducted in accordance with the IRB guidelines for experimental testing.
Experimental design and fMRI scanning procedure
During encoding, participants navigated a city consisting of eight stores randomly distributed around a circle in a virtual environment. They made a defined series of deliveries of a passenger to a store, with the spatial layout of the city uncorrelated with the temporal order of deliveries to ensure dissociation between the contextual elements of an episode (Fig. 1A). During retrieval, participants performed a spatiotemporal contextual retrieval task while being scanned. For each trial, participants were first asked whether they recognized a previously encountered store (item) compared to lure stores. To assay context memory, participants were presented with an additional question after each item recognition question regarding the contextual details associated with that item. Each trial was followed by a baseline task of variable duration based upon the jittered inter-stimulus interval (Fig. 1B). The experimental design is further described in Ekstrom et al. (2011), where this dataset was previously analyzed using a univariate approach.
The paradigm was configured as a mixed event-related, block design with 5 temporal and 5 spatial blocks that were interleaved for a total of 128 trials. Functional images were acquired on a Siemens 3 T TIM TRIO 32-channel scanner using a whole brain echo-planar imaging sequences (TR = 3 s, TE = 29 ms, slices = 47, field of view = 220 mm, flip angle = 90 degrees, bandwidth = 1684 Hz/pixel, voxel size = 2 × 2 × 2 mm3). A high-resolution (voxel size = 1 × 1 × 1 mm3) T1-weighted magnetization-prepared rapid acquisition with gradient echo (MP-RAGE) structural image was also acquired to register functional activations to anatomical images. Using Statistical Parametric Mapping (SPM8) software (Wellcome Centre for Neuroimaging, UCL, London), the data were high-pass filtered, motion-corrected and spatially smoothed with a 5 mm FWHM Gaussian kernel. Each individual participant's brain map was registered to the standard MNI template to carry out group-level analyses.
Region of interest/anatomical parcellation
Rather than apply the traditional seed-based functional connectivity approach where seeds, or clusters of voxels, are determined a priori based upon known task involvement, regions of interest (ROIs) relevant to retrieval and other processes, such as attention and working memory, were sampled throughout the entire brain. Other functional connectivity methods, such as voxelwise comparisons of the entire brain38, generate an unbiased, detailed profile of functional networks but encounter problems of multiple comparisons. The center of mass of twenty-six anatomical volumes delineated in standardized brain space50 was used to determine the coordinates of thirteen bilateral ROIs. The ROIs selected for this study depended on the defined masks in the atlas, resulting in restricted availability for some regions, such as retrosplenial cortex. Each ROI consisted of a 10 millimeter cube (a total of 125 voxels) assembled around the center of mass coordinate. If the constructed cubes encompassed voxels from surrounding regions, the cube was shifted in the x, y, or z coordinate directions to ensure the cube was comprised of voxels only belonging to the specified ROI. Finally, the hippocampus bilaterally was divided into anterior and posterior regions for a total of four ROIs, due to anatomical differences and functional specializations along the anterior to posterior axis51.
Statistical analysis and network construction
To assess inter-regional interactions throughout the brain, we employed a beta time series approach as described by Rissman and colleagues17. Briefly, each voxel's BOLD response in the task was modeled in a general linear model (GLM) as an individual regressor specifying the onset of each trial convolved with the canonical hemodynamic response function (HRF), resulting in 128 covariates of interest. The parameter, or beta, estimates derived for each trial for each voxel were then sorted by condition (spatial, temporal, correct, or incorrect trials) into a beta series. The spatial and temporal conditions consisted of both correct and incorrect retrieval questions to determine networks involved in the general process of spatiotemporal retrieval. The beta series of voxels belonging to an ROI were subsequently averaged culminating in twenty-six average beta series per condition. Finally, the Pearson product-moment correlation coefficient between all ROI's beta series was computed, creating a correlation matrix of all pairwise combinations (26 × 26) describing the strength of the functional relationship between two regions.
To deal with the false positives, we derived a distribution of correlation coefficient values by correlating randomly shuffled beta series 1,000 times and pooling across participants and all pairwise ROIs within a condition. All correlation values were transformed using Fisher's Z, or the arc hyperbolic tangent transform52. A functional connection, or edge, in the matrix was considered significant if the observed correlation value (correlation coefficient averaged across participants) was greater than the 90th percentile of the calculated distribution. This approach aimed to account for multiple comparisons and to fix the Type 1 error rate at 10%. This more liberal threshold preserves a higher network density thus providing enough connections to compute the graph theoretical metrics. Additional analyses using a bootstrap alpha level of 0.05 produced similar findings (Fig. S4) and suggest overall robust results. Additionally, the distribution of correlation values across all subjects and all pairwise regions was negatively skewed, so the average correlation value for all pairwise regions was greater than zero. Thus, we observed few negative correlations between regions, suggesting minimal “anti-correlated networks” within our findings. It is reasonable to expect that these areas might show mostly positive correlations in the first place because they have previously been implicated in memory processes.
Those connections that withstood our thresholding procedure were assigned a value of 1 in the adjacency matrix, a binary, undirected matrix of pairwise connections between all ROIs in each condition. To compare conditions, the set intersection of spatial and temporal (spatial + temporal) and correct and incorrect (correct + incorrect) yielded elements that were common to both pairs of conditions (Fig. S1, S2). In contrast, the set differences between the spatial and temporal conditions (spatial - temporal, temporal - spatial) and between the correct and incorrect conditions (correct - incorrect, incorrect - correct) revealed functional connections unique to a condition (Fig. 2, 4).
Networks were then constructed in two-dimensional space, preserving the anatomical distance between brain areas in the x and y coordinate directions. In order to facilitate visualization of the connectivity across the brain, these two-dimensional networks were overlaid on top of a transverse slice of a standardized brain. Lastly, node hierarchies were created for each network where nodes are plotted vertically in ascending node degree order; nodes on the same horizontal level have the same node degree. For all graphs, the colored circles indicate ROIs, while a line between two ROIs signifies a functional connection.
Behavioral measures
For the group level analyses, each individual participant's correlation coefficients were averaged to obtain a group correlation for each ROI pair and then the group correlation matrix was thresholded as outlined above. For our behavioral analysis, we derived each individual participants network by thresholding that participant's correlation matrix again using bootstrap resampling, which resulted in 16 individual networks. The density of each participant's correct retrieval network was then computed and subsequently regressed against their behavioral accuracy for all task trials. Next, using stepwise regression, a final multiple linear model was produced to account for the variance in the density data. This final model was based on an initial model consisting of a subset of nodes whose percent contribution of the overall network density individually showed a significant (p < 0.05) linear relationship with performance across participants. The algorithm then stepped through all 26 regressors (or nodes) to construct a final model that accounted for the most variance (diagramed in Fig. 3).
Network measures
The network topologies were characterized using measures of node degree, network density and betweenness centrality on binary, undirected networks53. Each measure was calculated from the Brain Connectivity Matlab toolbox (http://www.brain-connectivity-toolbox.net) or custom written Matlab code (Mathworks, Natick, MA). In the present study, a node, n, is defined as an ROI within the brain that has a defined anatomical location and volume within standard brain space and N is the set of all nodes. An edge is defined as a significant functional connection between two nodes, where aij is the connection status between node i and node j (i,j ∈ N). aij = 1 when there is a significant connection and aij = 0 when no connection is present. Node degree quantifies the total number of edges connected to a particular node:  . The density or total percent connectivity is the fraction of total number of edges in the network to the total number of possible edges:
. The density or total percent connectivity is the fraction of total number of edges in the network to the total number of possible edges:  . Finally, betweenness centrality is the sum of all the shortest paths that pass through the node of interest weighted by the inverse of the total number of shortest paths between two nodes:
. Finally, betweenness centrality is the sum of all the shortest paths that pass through the node of interest weighted by the inverse of the total number of shortest paths between two nodes:  , where ρhj is the shortest path or the fewest number of edges that can be traversed between two nodes. For simplicity, we averaged the betweenness centrality across hemispheres (Fig. 2D and Fig. 4D,E).
, where ρhj is the shortest path or the fewest number of edges that can be traversed between two nodes. For simplicity, we averaged the betweenness centrality across hemispheres (Fig. 2D and Fig. 4D,E).
References
Eichenbaum, H., Yonelinas, A. P. & Ranganath, C. The medial temporal lobe and recognition memory. Annu Rev Neurosci 30, 123–152 (2007).
Spiers, H. J. et al. Unilateral temporal lobectomy patients show lateralized topographical and episodic memory deficits in a virtual town. Brain 124, 2476–2489 (2001).
Konkel, A. & Cohen, N. J. Relational memory and the hippocampus: representations and methods. Front Neurosci 3, 166–174 (2009).
Konkel, A., Warren, D. E., Duff, M. C., Tranel, D. N. & Cohen, N. J. Hippocampal amnesia impairs all manner of relational memory. Front Hum Neurosci 2, 15 (2008).
Staresina, B. P. & Davachi, L. Mind the gap: binding experiences across space and time in the human hippocampus. Neuron 63, 267–276 (2009).
Ekstrom, A. D., Copara, M. S., Isham, E. A., Wang, W. C. & Yonelinas, A. P. Dissociable networks involved in spatial and temporal order source retrieval. Neuroimage 2011, 18 (2011).
Song, Z., Jeneson, A. & Squire, L. R. Medial temporal lobe function and recognition memory: a novel approach to separating the contribution of recollection and familiarity. J Neurosci 31, 16026–16032 (2011).
Johnson, M. K. Memory and reality. Am Psychol 61, 760–771 (2006).
Blumenfeld, R. S. & Ranganath, C. Prefrontal cortex and long-term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry 13, 280–291 (2007).
Berryhill, M. E., Phuong, L., Picasso, L., Cabeza, R. & Olson, I. R. Parietal lobe and episodic memory: bilateral damage causes impaired free recall of autobiographical memory. J Neurosci 27, 14415–14423 (2007).
Duarte, A., Henson, R. N., Knight, R. T., Emery, T. & Graham, K. S. Orbito-frontal cortex is necessary for temporal context memory. J Cogn Neurosci 22, 1819–1831 (2010).
Takahashi, N., Kawamura, M., Shiota, J., Kasahata, N. & Hirayama, K. Pure topographic disorientation due to right retrosplenial lesion. Neurology 49, 464–469 (1997).
Burgess, N., Maguire, E. A., Spiers, H. J. & O'Keefe, J. A temporoparietal and prefrontal network for retrieving the spatial context of lifelike events. Neuroimage 14, 439–453 (2001).
Watrous, A. J., Tandon, N., Conner, C. R., Pieters, T. & Ekstrom, A. D. Frequency-specific network connectivity increases underlie accurate spatiotemporal memory retrieval. Nature Neuroscience 16, 349–356 (2013).
Duarte, A., Ranganath, C. & Knight, R. T. Effects of unilateral prefrontal lesions on familiarity, recollection and source memory. J Neurosci 25, 8333–8337 (2005).
Cabeza, R. Introduction to the special issue on functional neuroimaging of episodic memory. Neuropsychologia 51, 2319–2321 (2013).
Rissman, J., Gazzaley, A. & D'Esposito, M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage 23, 752–763 (2004).
Sporns, O., Honey, C. J. & Kotter, R. Identification and classification of hubs in brain networks. PLoS One 2, e1049 (2007).
Kuhnert, M. T., Geier, C., Elger, C. E. & Lehnertz, K. Identifying important nodes in weighted functional brain networks: a comparison of different centrality approaches. Chaos 22 (2012).
van den Heuvel, M. P. & Sporns, O. Network hubs in the human brain. Trends Cogn Sci 17, 683–696 (2013).
Ranganath, C. et al. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia 42, 2–13 (2004).
Rugg, M. D. et al. Item memory, context memory and the hippocampus: fMRI evidence. Neuropsychologia 50, 3070–3079 (2012).
Davachi, L. & Wagner, A. D. Hippocampal contributions to episodic encoding: insights from relational and item-based learning. J Neurophysiol 88, 982–990 (2002).
Diana, R. A., Yonelinas, A. P. & Ranganath, C. Medial Temporal Lobe Activity during Source Retrieval Reflects Information Type, not Memory Strength. J Cogn Neurosci 22, 1808–1822 (2009).
Eldridge, L. L., Engel, S. A., Zeineh, M. M., Bookheimer, S. Y. & Knowlton, B. J. A dissociation of encoding and retrieval processes in the human hippocampus. J Neurosci 25, 3280–3286 (2005).
Daselaar, S. M., Fleck, M. S. & Cabeza, R. Triple dissociation in the medial temporal lobes: recollection, familiarity and novelty. J Neurophysiol 96, 1902–1911 (2006).
Davachi, L., Mitchell, J. P. & Wagner, A. D. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci U S A 100, 2157–2162 (2003).
Suzuki, M. et al. Neural basis of temporal context memory: a functional MRI study. Neuroimage 17, 1790–1796 (2002).
Johnson, J. D., McDuff, S. G., Rugg, M. D. & Norman, K. A. Recollection, familiarity and cortical reinstatement: a multivoxel pattern analysis. Neuron 63, 697–708 (2009).
Shallice, T. et al. Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature 368, 633–635 (1994).
St Jacques, P., Rubin, D., LaBar, K. S. & Cabeza, R. The short and long of it: neural correlates of temporal-order memory for autobiographical events. (2008).
Nyberg, L. et al. General and specific brain regions involved in encoding and retrieval of events: what, where and when. P Natl Acad Sci USA 93, 11280–11285 (1996).
Cabeza, R. et al. Brain regions differentially involved in remembering what and when: a PET study. Neuron 19, 863–870 (1997).
Johnson, J. D., Suzuki, M. & Rugg, M. D. Recollection, familiarity and content-sensitivity in lateral parietal cortex: a high-resolution fMRI study. Front Hum Neurosci 7, 219 (2013).
Elman, J. A., Cohn-Sheehy, B. I. & Shimamura, A. P. Dissociable parietal regions facilitate successful retrieval of recently learned and personally familiar information. Neuropsychologia 51, 573–583 (2013).
Fletcher, P. C. et al. Brain systems for encoding and retrieval of auditory-verbal memory. An in vivo study in humans. Brain 118 (Pt 2), 401–416 (1995).
Wagner, A. D., Shannon, B. J., Kahn, I. & Buckner, R. L. Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Sciences 9, 445–453 (2005).
Turk-Browne, N. B. Functional interactions as big data in the human brain. Science 342, 580–584 (2013).
Nadel, L., Samsonovich, A., Ryan, L. & Moscovitch, M. Multiple trace theory of human memory: computational, neuroimaging and neuropsychological results. Hippocampus 10, 352–368 (2000).
Norman, K. A. & O'Reilly, R. C. Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychol Rev 110, 611–646 (2003).
Watrous, A. & Ekstrom, A. The spectro-contextual encoding and retrieval theory of episodic memory. Frontiers in human neuroscience 8, 75 (2014).
Mitchell, K. J. & Johnson, M. K. Source monitoring 15 years later: what have we learned from fMRI about the neural mechanisms of source memory? Psychol Bull 135, 638–677 (2009).
Azab, M., Stark, S. M. & Stark, C. E. Contributions of the human hippocampal subfields to spatial and temporal pattern separation. Hippocampus 24, 293–302 (2014).
Kraus, B. J., Robinson, R. J., 2nd, White, J. A., Eichenbaum, H. & Hasselmo, M. E. Hippocampal “time cells”: time versus path integration. Neuron 78, 1090–1101 (2013).
Gilbert, P. E., Kesner, R. P. & Lee, I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus 11 (2001).
Nadel, L. & Moscovitch, M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol 7, 217–227 (1997).
Teyler, T. J. & DiScenna, P. The hippocampal memory indexing theory. Behav Neurosci 100, 147–154 (1986).
Tulving, E. Episodic memory: from mind to brain. Annu Rev Psychol 53, 1–25 (2002).
Wang, L., Saalmann, Y. B., Pinsk, M. A., Arcaro, M. J. & Kastner, S. Electrophysiological low-frequency coherence and cross-frequency coupling contribute to BOLD connectivity. Neuron 76, 1010–1020 (2012).
Tzourio-Mazoyer, N. et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289 (2002).
Poppenk, J. & Moscovitch, M. A hippocampal marker of recollection memory ability among healthy young adults: contributions of posterior and anterior segments. Neuron 72, 931–937 (2011).
Fischer, R. A. The General Sampling Distribution of the Multipe Correlation Coefficient. Proceedings of the Royal Society of Mathematical, Physical and Engineering Sciences A 121, 654–673 (1928).
Rubinov, M. & Sporns, O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52, 1059–1069 (2010).
Acknowledgements
This work was supported by NINDS R01NS076856, the Sloan Foundation and the Hellman Young Investigator Award. We thank Emilio Ferrer and the UC Davis memory group for comments on this manuscript.
Author information
Authors and Affiliations
Contributions
A.S. analyzed the data, prepared all figures and wrote the main manuscript text. M.C. designed the experimental paradigm, collected data from participants and preprocessed the fMRI data. A.W. assisted in the analyses and provided scripts used in creating figures. A.E. conceived and supervised the project, designed the experimental paradigm and wrote the main manuscript text. All authors read and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Schedlbauer, A., Copara, M., Watrous, A. et al. Multiple interacting brain areas underlie successful spatiotemporal memory retrieval in humans. Sci Rep 4, 6431 (2014). https://doi.org/10.1038/srep06431
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep06431
This article is cited by
-
From cognitive maps to spatial schemas
Nature Reviews Neuroscience (2023)
-
The distinct disrupted plasticity in structural and functional network in mild stroke with basal ganglia region infarcts
Brain Imaging and Behavior (2022)
-
Elaboration Benefits Source Memory Encoding Through Centrality Change
Scientific Reports (2019)
-
Development of spatial orientation skills: an fMRI study
Brain Imaging and Behavior (2019)
-
Widespread theta synchrony and high-frequency desynchronization underlies enhanced cognition
Nature Communications (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.