Abstract
Inflammatory macrophages in colonic mucosa are the leading drivers of the pathology associated with inflammatory bowel disease (IBD). Here we examined whether gadolinium chloride (GdCl3), a macrophage selective inhibitor, would improve the course of 2,4,6-trinitro benzene sulfonic acid (TNBS) and dextran sodium sulfate (DSS)-induced colitis in mice and the potential mechanisms were investigated. By giving GdCl3 to colitis mice through intravenous or intrarectal route, we found that GdCl3 markedly ameliorated the colitis severity, including less weight loss, decreased disease activity index scores and improved mucosal damage. To investigate the potential mechanisms, flow-cytometric analysis was performed to detect the proportion of mucosal macrophages in colon. The results showed that GdCl3 had no macrophage depletion effect in colonic mucosa, but significantly suppressed TNBS and DSS-induced TNFα, IL-1β and IL-6 secretions. Also, Western blotting analysis indicated that NF-κB p65 expression was significantly attenuated in the mucosa in colitis mice with GdCl3 treatment. Then, the anti-inflammatory activity of GdCl3 was confirmed in LPS-stimulated RAW 264.7 cells that GdCl3 might down-regulate the production of proinflammatory cytokines by macrophages through inhibition of the NF-κB signaling pathway. Therefore, intervention with mucosal inflammatory macrophages may be a promising therapeutic target in IBD.
Similar content being viewed by others
Introduction
Inflammatory bowel disease (IBD) comprises Crohn's disease and ulcerative colitis that cause chronic and remittent-relapsing intestinal inflammation of all or part of the intestinal tract1,2. Although the aetiology of IBD remains unclear, it is thought to result from dysregulation of the mucosal immune responses to intestinal bacterial antigens in genetically predisposed individuals1,3. Currently, treatment options for IBD are mainly focused on suppressing mucosal immune responses, including the use of 5-aminosalicylic acid (5-ASA) agents, steroids, antimicrobials and some immunomodulators4. However, although they are reasonably successful in many patients, still a great number cannot achieve remission, highlighting the need for novel therapeutic targets.
Infiltration and activation of macrophages in colon are central features of IBD and inflammatory macrophages in mucosa are thought to play an essential role in the pathogenesis of IBD2,5,6,7. In IBD and experimental colitis, monocytes in blood are recruited to the mucosa and differentiate into activated macrophages that produce pro-inflammatory cytokines, such as tumor necrosis factor α (TNFα), interleukin (IL)-1 and IL-62,7,8. Activation of NF-κB is thought as a strong inducer of these proinflammatory cytokine expressions9,10 and activated NF-κB has been demonstrated in colonic macrophages of patients with IBD. Alteration in cytokine production by macrophages is one major component of the pathology of IBD. Therefore, strategies for targeting inflammatory mucosal macrophages may be important for developing new therapeutics. However, studies on intervention with mucosal macrophages in colon are really limited, which might mainly due to the limited intervention methods.
Gadolinium chloride (GdCl3), a rare earth metal, is widely used experimentally11,12. The role of GdCl3 in macrophage elimination has been widely proven in liver of experimental animals, indicating a preventive or therapeutic effect in liver injury12,13,14. GdCl3 is also employed as a macrophage selective inhibitor in vivo11. It has been reported that GdCl3 has no depletion effect in tissue macrophages in lung, but it decreases the expression of TNFα and IL-6 after LPS stimulation in rat15. However, the effect of GdCl3 on mucosal macrophages in colon remains largely unknown.
Experimental animal models are crucial tools that provide a road map allowing us to probe the pathogenesis of diseases and to test emerging therapeutic strategies. 2,4,6-trinitro benzene sulfonic acid (TNBS) and dextran sodium sulfate (DSS) colitis models are canonical IBD models that the onset of inflammation is immediate and the procedure is relatively straightforward; therefore, the two models have been frequently used to evaluate the efficacy of potential therapeutic agents16,17.
The present study was performed to investigate the protective role and the potential mechanisms of GdCl3 in TNBS- and DSS-induced colitis. We studied the elimination effect of GdCl3 on mucosal macrophages. The colitis severity that included body weight loss, disease activity index scores and mucosal damage, was evaluated and the protein secretion of cytokines and the activity of NF-κB signal pathways were also studied in colitis mice treated with GdCl3. Also, the role of GdCl3 in macrophages was further investigated in activated RAW 264.7 cells in vitro.
Results
GdCl3 has no elimination effect on mucosal macrophages in colon in mice
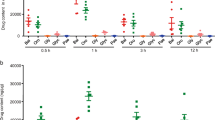
The effect of GdCl3 on mucosal macrophages in colon is still unknown. In this study, we evaluated the proportion of mucosal macrophages in colon in mice after intravenous administration of GdCl3. It was revealed that intravenous treatment of GdCl3 at each concentration could not deplete macrophages in colonic mucosa. We then used GdCl3 at a concentration of 10 mg/kg body weight for 1 day before animal treatment for further study in accordance with the method described previously15,18.
Figure 1A showed that the proportion of macrophages in colonic mucosa was not altered after GdCl3 treatment (10 mg/kg body weight) for 1 day in mice (9.76 ± 0.31% vs 9.83 ± 0.15%, p > 0.05). No difference in the proportion of CD3+ cells in colonic mucosa was observed between GdCl3-treated mice and control mice (p > 0.05; Figure 1B). In serum and colonic mucosa tissue, the expression profiles of TNFα, IL-1β and IL-6 were not obviously elevated after GdCl3 treatment (p > 0.05 for all three cytokine types; Figures 1C, D). Thus, GdCl3 could not deplete macrophages in colonic mucosa and also would not induce inflammation in vivo.
GdCl3 had no macrophage depletion effect in colonic mucosa.
After intravenous treatment of GdCl3 (10 mg/kg body weight) for 1 day, mice were killed and the proportion of mucosal macrophages in colon and the cytokine expression levels in serum and colonic mucosa were evaluated. (A) Proportion of mucosal macrophages in colon. (B) Proportion of CD3+ cells in colonic mucosa. (C) Cytokine expression of TNFα, IL-1β and IL-6 in serum. (D) Cytokine levels of TNFα, IL-1β and IL-6 in colonic mucosa. (ns: no significance).
GdCl3 is protective against colitis induced by TNBS and DSS
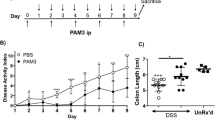
To evaluate the potential role of GdCl3 in colitis in vivo, we used two murine models of colitis induced by TNBS and DSS. In TNBS colitis, GdCl3 was given to mice on day 3 of TNBS application intravenously. The mortality of TNBS colitis mice was improved by about 20% after GdCl3 treatment (Figure 2A). Treatment with GdCl3 resulted in prominent protection from colitis as assessed by body weight, disease activity index (DAI) scores, colon length and histopathological damage of the colon. Control mice presented weight loss after TNBS administration, whilst colitis mice treated with GdCl3 showed markedly less body weight loss (Figure 2B). GdCl3 significantly improved DAI scores and shortening of colon length in TNBS colitis (Figures 2C, D). Histopathological analysis of the colon, examined on day 7 and 14, showed marked crypt architecture damage, inflammatory cell infiltration and ulceration in colitis mice. GdCl3 treatment significantly improved these damages of colon as assessed by the histopathological scores (Figure 2E).
GdCl3 was protective against colitis in TNBS-treated mice.
After induction of TNBS colitis, GdCl3 was given to mice intravenously on day 3. After colitis induction for 7 days or 14 days, mice were killed and the colitis severity was evaluated. (A) The survival rate. (B) The percentage of initial body weight. (C) The disease activity index (DAI). (D) The colon length. (E) The histopathological evaluation. (*P < 0.05, **P < 0.01, ***P < 0.001; ##P < 0.01, ###P < 0.001 comparing TNBS + GdCl3 to TNBS).
Intrarectal treatment of GdCl3 began on day 3 of TNBS application and continued until day 7. Colitis was also significantly ameliorated after GdCl3 administration as assessed by changes of body weight, DAI scores and mucosal damage, which were similar to mice treated with GdCl3 intravenously and there was no significant difference between mice treated with GdCl3 through intravenous route and intrarectal route.
Mice were exposed to 3% DSS in drinking water for 7 days in DSS colitis. GdCl3 was administrated to mice through either intravenous or intrarectal route similar to the treatment in TNBS colitis mice. The results revealed that GdCl3 treatment also resulted in striking protection from DSS-induced colitis (Table 1).
GdCl3 suppresses TNBS- and DSS-induced proinflammatory cytokine secretions
To evaluate whether the protection from colitis induced by TNBS and DSS in mice with GdCl3 treatment was associated with a reduction in the production of proinflammatory cytokines, expression levels of TNFα, IL-1β and IL-6 in serum and colonic mucosa of vehicle and mice treated with GdCl3 were detected using ELISA.
In TNBS colitis mice treated with GdCl3 intravenously, the proportion of macrophages and CD3+ cells in colonic mucosa was not altered on both day 7 and day 14 (Figures 3A, B). On day 7, TNFα, IL-1β and IL-6 protein levels were significantly increased in serum and colonic mucosa obtained from mice treated with TNBS (Figures 3C, D). On day 14, expression profiles of TNFα, IL-1β and IL-6 were a little down-regulated (Figures 3C, D). But GdCl3 treatment significantly reduced TNFα, IL-1β and IL-6 levels in serum and colonic mucosa on day 7 as compared to those of vehicle control mice (Figures 3C, D). A significant suppression by GdCl3 treatment of TNFα, IL-1β and IL-6 levels in serum and colonic mucosa was also noted on day 14 (Figures 3C, D).
GdCl3 treatment reduced TNBS-induced TNFα, IL-1β and IL-6 expressions.
After induction of TNBS colitis, GdCl3 was given to mice intravenously on day 3. And mice were killed on day 7 or day 14. The amount of mucosal macrophages in colon and the levels of cytokines in serum and colonic mucosa were evaluated. (A) Proportion of mucosal macrophages in colon. (B) Proportion of mucosal CD3+ cells in colon. (C) Cytokine expression of TNFα, IL-1β and IL-6 in serum. (D) Cytokine levels of TNFα, IL-1β and IL-6 in colonic mucosa. (Ctrl, control; G, GdCl3) (ns: no significance; **P < 0.01, ***P < 0.001; #P < 0.05, ##P < 0.01, ###P < 0.001 comparing TNBS + GdCl3 to TNBS).
The proportion of mucosal macrophages and CD3+ cells in colon was also not changed in TNBS colitis mice treated with GdCl3 intrarectally. TNBS-induced expression levels of TNFα, IL-1β and IL-6 were significantly decreased after intrarectal administration of GdCl3, which were not different from those in mice treated with GdCl3 intravenously.
In DSS colitis mice, GdCl3 was administrated to mice through either intravenous or intrarectal route. The results indicated that GdCl3 also showed an anti-inflammatory property in serum and colonic mucosa (Table 1).
GdCl3 reduces proinflammatory cytokine production by LPS-stimulated RAW 264.7 cells
The anti-inflammatory activity of GdCl3 was also confirmed in LPS-stimulated RAW 264.7 cells in vitro. To define the experimental dose range of GdCl3 for in vitro use, its effect on cell viability was assessed by MTT assay. GdCl3 did not exert a toxicity in RAW 264.7 cells at a concentration ranging from 10 to 200 μM (Figure 4A). Flow-cytometric analysis also showed a lack of pro-apoptotic effect of GdCl3 on the cell (data not shown). GdCl3 at a concentration of 100 μM was used for further studies based on a previous study19. In RAW 264.7 cells, TNFα, IL-1β and IL-6 production was markedly increased in the culture supernatant after LPS stimulation, whereas the levels of these proinflammatory cytokines were significantly reduced by GdCl3 (Figures 4C, D and E).
GdCl3 decreased proinflammatory cytokine production in LPS-stimulated RAW264.7 cells.
RAW264.7 cells were treated with GdCl3 in various concentrations (0–200 μM) for required incubation times to evaluate the cell viability. And then cells were stimulated with LPS (100 ng/mL) with or without GdCl3 (100 μM). At the end of incubation, the production of cytokines was determined. (A) Changes in cell viability. (B) Cytokine production of TNFα. (C) Cytokine production of IL-1β. (D) Cytokine production of IL-6. (*P < 0.05, **P < 0.01).
GdCl3 suppresses NF-κB activation in mucosa in colitis mice and also in LPS-stimulated RAW264.7 cells
Nuclear factor kappa B (NF-κB), a transcription factor, plays an essential role in inflammation. Activation of macrophages is regulated by NF-κB activation20. In TNBS colitis mice, the NF-κB p65 expression in colonic mucosa was significantly attenuated with GdCl3 treatment through intravenous route on both day 7 and day 14. (Figures 5A and B) Intrarectal administration of GdCl3 also decreased the NF-κB p65 expression in colonic mucosa in TNBS colitis mice, which was similar to mice treated with GdCl3 intravenously. In DSS colitis mice, NF-κB activation in mucosa was also suppressed with GdCl3 treatment. Also, in vitro studies showed that GdCl3 markedly reduced NF-κB p65 expression in LPS-stimulated RAW 264.7 cells (Figure 5B). Therefore, GdCl3 may down-regulate the secretion of proinflammatory cytokines by macrophages through suppression of NF-κB activation.
GdCl3 reduced NF-κB p65 expression in mucosa in colitis mice and also in LPS-stimulated RAW264.7 cells.
(A) Western blot analysis of protein level of NF-κB p65 in colitis mice. (B) Western blot analysis of protein level of NF-κB p65 in LPS-stimulated RAW264.7 cells. (Ctrl, control; G, GdCl3) (ns: no significance; *P < 0.05, **P < 0.01, ***P < 0.01; #P < 0.05, ##P < 0.01, ###P < 0.001 comparing TNBS + GdCl3 to TNBS in (A); ##P < 0.01, ###P < 0.001 comparing to the control in (B)).
Discussion
IBD is a chronic inflammatory disease with rising incidence worldwide and alteration in cytokine production by inflammatory macrophages is one major component. GdCl3 is a macrophage selective inhibitor and has been proved to exert anti-inflammatory effect in lung and liver11,15. Here, for the first time, we showed a protection effect on colitis severity through an anti-inflammatory property of GdCl3 in colonic mucosa in vivo by suppressing the proinflammatory cytokine secretions by macrophages.
In this study, we demonstrated a protection against colitis induced by TNBS and DSS in mice with GdCl3 treatment, exhibiting improved weight loss, DAI scores and mucosal damage. To investigate the protective mechanism of GdCl3 in colitis, we first evaluated whether GdCl3 had a macrophage eliminating role in mucosal macrophages in colon in mice. Previous studies had indicated that GdCl3 treatment efficiently depleted Kupffer cells in the liver11,21,22. Wehner, et al.23 had showed that intravenous treatment with chlodronate liposomes and GdCl3 led to a significant depletion of 52% muscularis macrophages in colon in rat. However, the result of our study showed an absence of macrophage depletion effect of GdCl3 in colonic mucosa in mice.
To evaluate whether the protection against colitis was associated with down-regulated production of proinflammatory cytokines, expression levels of TNFα, IL-1β and IL-6 in serum and colonic mucosa of vehicle and mice treated with GdCl3 were detected. Kono, et al.15 reported that GdCl3 had no depletion effect in tissue macrophages in lung, but it decreased the expression of TNFα and IL-6 in lung after LPS stimulation in rat. Results of our study revealed that the level of TNFα, IL-1β and IL-6 in colonic mucosa was markedly reduced in colitis mice with GdCl3 treatment. Thus, GdCl3 had an anti-inflammatory effect in colonic mucosa in colitis mice, possibly through suppressing the proinflammatory cytokine secretion by inflammatory macrophages.
It has been reported that the role of GdCl3 in lung injury might owe to the inhibition of production of inflammatory mediators by the Kupffer cells15. In our study, it was revealed that intravenous administration of GdCl3 induced remission in colitis mice. Thus it was possible that not only the reduced proinflammatory cytokine production by macrophages in mucosa but also the inhibition of production of inflammatory mediators by the Kupffer cells played a role in the remission of colitis. However, the present study revealed that GdCl3 treatment through intrarectal route showed a similar colitis-protective effect in colitis mice and no significant difference was found in colitis mice treated with GdCl3 through the two routes. These results further supported that GdCl3 induced remission in colitis mice through reducing proinflammatory cytokine production by inflammatory macrophages in colonic mucosa.
The anti-inflammatory property of GdCl3 was also confirmed in vitro in RAW 264.7 cells with LPS stimulation. Results of our study indicated that GdCl3 exerted no cytotoxic or pro-apoptotic effect in RAW264.7 cells, which is consistent with a previous report19. Production of TNFα, IL-1β and IL-6 was markedly reduced in the culture supernatant in LPS-stimulated cells with GdCl3 treatment. Therefore, GdCl3 also had an anti-inflammatory effect on activated macrophages in vitro, down-regulating the production of proinflammatory cytokines.
Furthermore, western blot analysis showed that the expression of NF-κB p65 was significantly attenuated in colonic mucosa in colitis mice treated with GdCl3. In in vitro studies, GdCl3 also decreased NF-κB p65 expression in activated RAW 264.7 cells. Thus, GdCl3 may improve the colitis severity through down-regulated secretion of proinflammatory cytokines in macrophages via inactivation of NF-κB signal pathway.
In summary, GdCl3 markedly improves the colitis severity in experimental colitis through suppression of NF-κB activation with reduced production of proinflammatory cytokines by mucosal macrophages in colon. Therefore,it provides us a new and bright prospect of promising therapeutics in IBD.
Methods
Animal treatment
Male C57BL/6J mice weighing 20–25 g (aged 8–10 weeks) were used in this study. All mice were purchased from Beijing HFK Bioscience Company. Animals were housed on a temperature- (20 ± 1°C) and light-controlled cycle (12 hours) with free access to standard laboratory chow and tap water. All procedures were approved by the Animal Care and Use Committee of Shandong University and were performed in accordance with the Animal Management Rules of the Chinese Ministry of Health.
GdCl3 (5, 10, 15 20 mg/kg body weight; dissolved in 0.1 mL PBS as a vehicle) was administrated to mice via the tail vein. The PBS vehicle (0.1 mL) was administrated as a control. Mice were sacrificed for detection of the amount of mucosal macrophages in colon after GdCl3 treatment for 1 day, 2 days and 3 days.
Colitis induction and design of treatment
Two well-established colitis models were used in this study. The TNBS colitis was induced by rectal administration of TNBS (2 mg in 50% ethanol, 0.1 mL in total) (Sigma-Aldrich, St. Louis, MO) via a polyethylene catheter inserted 2 to 3 cm from the anus. An equivalent volume of PBS was instilled into control mice. The DSS colitis was induced by an intake of 3% (w/v) DSS (40,000 MW) (MP Biomedicals, Solon, OH) dissolved in drinking water for 7 consecutive days and then was replaced with regular water for another 7 days. Control mice received only drinking water. GdCl3 (10 mg/kg body weight) was given to mice on day 3 of TNBS and DSS application through intravenous route or begun on day 3 of TNBS and DSS application and continued until day 7 through intrarectal route. Control mice received equivalent administration of PBS. Mice were sacrificed on day 7 or day 14 after induction of colitis.
Evaluation of colitis progression
Body weights were recorded daily. Severity of colitis was assessed by the disease activity index (DAI) based on weight loss, stool bleeding and stool consistency in accordance with the method described previously24,25. The DAI was scored on a scale of 0–4 for each parameter and then summed up for each mouse and each group.
Mice were killed on day 7 or day 14 with colons removed. Colons were measured and cut into sections. Histopathological studies were performed on paraffin-embedded, 4 μm thick distal colon sections, stained with haematoxylin and eosin. Histology was scored microscopically in a blinded fashion26,27 as shown in Table 2.
Flow cytometric analysis of mucosal macrophages in colon
Colons were dissected from euthanized mice and the fecal contents and mesenteric tissues were removed. Under a Leitz dissection microscope, the colonic mucosa was gently peeled off the underlying layers, using a pair of fine dissection forceps. The mucosa were then cut into pieces of about 5 mm and placed into 10 mg·mL−1 collagenase I in DMEM supplemented with 10% FBS at 37°C for 60 min. At the end of the reaction, the enzymatic action was blocked with 5 mm EDTA at 37°C for 10 min. The suspension was filtered on nylon mesh (70 μm) and cells were harvested after centrifugation for 5 min at 1000 g and resuspended in PBS. The cells were incubated for 20 min at 4°C in the dark with the antibodies and then were detected using a BD flow cytometer. The following antibody clones were used: F4/80-FITC, which had been used previously24,28 and CD3-FITC from eBiosciense (San Diego, CA).
Measurement of cytokine levels in serum and colonic mucosa
After collection, peripheral blood was centrifuged for 5 min at 1500 g and serum was collected. Colonic mucosa was cut into pieces and was initially homogenised in a prepared ice-cold 100 mm Tris-HCl buffer, pH 7.0, containing a cocktail of protease inhibitors (Beyotime, Shanghai, China) supplemented with 1 mm phenylmethanesulfonyl fluoride. Levels of TNFα, IL-1β and IL-6 in serum and colonic mucosa were measured by ELISA kits (KYM, Beijing, China) according to the manufacture's recommendations.
Cell culture and treatment
RAW264.7 cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM, Gibco by Invitrogen, CA, USA), containing 10% fetal bovine serum (FBS, Gibco). Cells were seeded onto the 96-well plate with 5.0 × 103 cells per well. After 12 hours, cells were treated with gadolinium chloride (GdCl3, Sigma-Aldrich, St. Louis, MO) in various concentrations (0–200 μM) for required incubation times (24 hours, 48 hours and 72 hours).
Cell viabilities and apoptosis of RAW 264.7 cells with GdCl3 treatment
At the end of incubation of GdCl3 in RAW264.7 cells, 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazo-lium bromide (MTT, Amresco, Solon, OH, USA) in PBS was added to each well to reach a final concentration of 0.5 mg/mL and the cells were further incubated at 37°C for 4 hours. Then the supernatant was removed and 150 μL DMSO was added to dissolve the formazan. Absorbance was measured at 490 nm on a microplate reader (ThermoFisher Scientific, San Jose, CA). The viabilities of treated cells were expressed as the percentage of control cells, which was assumed to be 100%.
After treatment with GdCl3 for 24 hours, RAW264.7 cells were trypsinized. The cell pellets were obtained after centrifugation at 1000 rpm for 5 min. Cells were washed twice with cold PBS and then were resuspended in 100 μL 1 × Binding buffer at a concentration of 1.0 × 106 cells/mL. Staining of FITC Annexin V and PI was according to the manufacture's instruction (FITC Annexin V apoptosis detection kit II, BD pharmingen™, San Diego, CA). The apoptotic rate was analyzed by flow cytometry.
LPS treatment and cytokine analysis
Cells (3.0 × 104) in 500 μL medium were added to 24-well plates. After 12 hours, LPS at a concentration of 100 ng/mL with or without GdCl3 (100 μM) was added to the wells. The supernatants were collected 24 hours, 48 hours and 72 hours after stimulation. The production levels of TNFα, IL-1β and IL-6 in the supernatant were detected by ELISA.
Western blot analysis
Total protein was extracted from mucosal samples of mice and from RAW 264.7 cell lysates in radioimmunoprecipitation assay (RIPA) buffer (Beyotime Institute of Biotechnology, Shanghai). Protein was quantified by using a BCA protein quantification kit (Beyotime). An amount of 20 μg total protein from each sample was separated by sodium dodecyl sulfate–polyacrylamide gel electro phoresis and transferred to a polyvinylidene difluoride membrane (0.22 μm pore; Millipore, Bedford, MA, USA). After being blocked with 5% skim milk powder diluted in TBS containing 0.1% Tween-20 for 1 h, the membrane was incubated with primary antibodies (anti- NF-κB p65 monoclonal antibody, Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C overnight. Horseradish peroxidase– conjugated secondary antibodies (Zhongshan Gold Bridge, Beijing, China) were probed the next day and an enhanced chemiluminescent substrate (Millipore) was used to detect the protein bands. Densitometry of protein bands was quantified by use of Quantity One 4.6.2 (Bio-Rad Laboratories, Hercules, CA, USA).
Statistical analysis
In mice, the nonparametric Mann-Whitney test was used to determine statistical differences between two groups. One-way ANOVA was performed to compare three groups. If the ANOVA analysis was significant, the Newman-Keuls test was applied for comparison between each two groups. In RAW264.7 cells, cytokine concentrations and NF-κB p65 expressions were compared using the Student's t test. All data were analyzed with GraphPad Prism 5.01 (Graphpad Software, San Diego, CA, USA). Differences were considered statistically significant at P < 0.05.
References
Podolsky, D. K. Inflammatory bowel disease. N Engl J Med Vol. 347, 417–429 (2002).
Maloy, K. J. & Powrie, F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 474, 298–306 (2011).
Xavier, R. J. & Podolsky, D. K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448, 427–434 (2007).
Baumgart, D. C. & Sandborn, W. J. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet 369, 1641–1657 (2007).
Zhang, S., Liu, X., Wang, H., Peng, J. & Wong, K. K. Silver nanoparticle-coated suture effectively reduces inflammation and improves mechanical strength at intestinal anastomosis in mice. J Pediatr Surg 49, 606–613 (2014).
Bain, C. C. & Mowat, A. M. Intestinal macrophages - specialised adaptation to a unique environment. Eur J Immunol 41, 2494–2498 (2011).
Bain, C. C. et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6C(hi) monocyte precursors. Mucosal Immunol 6, 498–510 (2012).
Zhang, Y. et al. Berberine Hydrochloride Prevents Post-Surgery Intestinal Adhesion and Inflammation in Rats. J Pharmacol Exp Ther 349, 417–426 (2014).
Andresen, L. et al. Activation of nuclear factor kappaB in colonic mucosa from patients with collagenous and ulcerative colitis. Gut 54, 503–509 (2005).
Schreiber, S. The complicated path to true causes of disease: role of nuclear factor kappaB in inflammatory bowel disease. Gut 54, 444–445 (2005).
Adding, L. C., Bannenberg, G. L. & Gustafsson, L. E. Basic experimental studies and clinical aspects of gadolinium salts and chelates. Cardiovasc Drug Rev 19, 41–56 (2001).
Jiang, S., Gavrikova, T. A., Sharifov, O. F. & Messina, J. L. Role of tissue macrophages in the development of critical illness diabetes. Shock 37, 70–76 (2012).
Hildebrand, F. et al. Kupffer cells and their mediators: the culprits in producing distant organ damage after trauma-hemorrhage. Am J Pathol 169, 784–794 (2006).
Montosi, G. et al. Kupffer cells and macrophages are not required for hepatic hepcidin activation during iron overload. Hepatology 41, 545–552 (2005).
Kono, H. et al. Role of Kupffer cells in lung injury in rats administered endotoxin 1. J Surg Res 129, 176–189 (2005).
Strober, W., Fuss, I. J. & Blumberg, R. S. The immunology of mucosal models of inflammation. Annu Rev Immunol 20, 495–549 (2002).
Wirtz, S., Neufert, C., Weigmann, B. & Neurath, M. F. Chemically induced mouse models of intestinal inflammation. Nat Protoc 2, 541–546 (2007).
Gill, S. S. et al. Role of pulmonary intravascular macrophages in endotoxin-induced lung inflammation and mortality in a rat model. Respir Res 9, 69 (2008).
Gou, B. D., Bian, S., Zhang, T. L. & Wang, K. Gadolinium-promoted precipitation of calcium phosphate is associated with profibrotic activation of RAW 264.7 macrophages. Toxicol In Vitro 24, 1743–1749 (2010).
Rogler, G. et al. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology 115, 357–369 (1998).
Hardonk, M. J., Dijkhuis, F. W., Hulstaert, C. E. & Koudstaal, J. Heterogeneity of rat liver and spleen macrophages in gadolinium chloride-induced elimination and repopulation. J Leukoc Biol 52, 296–302 (1992).
Ardelean, D. S. et al. Anti-VEGF therapy reduces intestinal inflammation in Endoglin heterozygous mice subjected to experimental colitis. Angiogenesis 17, 641–659 (2014).
Su, X. L. et al. [Shen warming Pi strengthening method intervened IBS-D rats: an efficacy assessment]. Zhongguo Zhong Xi Yi Jie He Za Zhi 34, 197–202 (2014).
Funakoshi, T. et al. A novel NF-kappaB inhibitor, dehydroxymethylepoxyquinomicin, ameliorates inflammatory colonic injury in mice. J Crohns Colitis 6, 215–225 (2012).
Qualls, J. E., Kaplan, A. M., van Rooijen, N. & Cohen, D. A. Suppression of experimental colitis by intestinal mononuclear phagocytes. J Leukoc Biol 80, 802–815 (2006).
Hughes, P. A. et al. Post-inflammatory colonic afferent sensitisation: different subtypes, different pathways and different time courses. Gut 58, 1333–1341 (2009).
Reinecke, K. et al. The JNK inhibitor XG-102 protects against TNBS-induced colitis. PLoS One 7, e30985 (2012).
Heidemann, J., Kebschull, M., Tepasse, P. R. & Bettenworth, D. Regulated expression of leukocyte-specific transcript (LST) 1 in human intestinal inflammation. Inflamm Res 63, 513–517 (2014).
Acknowledgements
The authors appreciate the considerable assistance from the Key Laboratory of Cardiovascular Remodeling and Function Research in the Qilu Hospital of Shandong University. This work was supported by the National Natural Science Foundation of China (81270457 and 81170352) and the scientific research foundation for outstanding young scientist of Shandong Province (BS2012SW012).
Author information
Authors and Affiliations
Contributions
C.D. performed the experiments, analyzed the data and wrote the paper. P.W., Y.B.Y., F.X.C. and J.L. performed the experiments and analyzed the data. Y.Q.L. provided advice in designing experiments and writing the paper. All authors have reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Du, C., Wang, P., Yu, Y. et al. Gadolinium chloride improves the course of TNBS and DSS-induced colitis through protecting against colonic mucosal inflammation. Sci Rep 4, 6096 (2014). https://doi.org/10.1038/srep06096
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep06096
This article is cited by
-
Electroacupuncture intervention of visceral hypersensitivity is involved in PAR-2-activation and CGRP-release in the spinal cord
Scientific Reports (2020)
-
Gadolinium chloride attenuates acetic acid-evoked colitis in mice by reducing neutrophil infiltration and pro-oxidative enzyme activity
Naunyn-Schmiedeberg's Archives of Pharmacology (2019)
-
Assessment of Serum sTREM-1 as a Marker of Subclinical Inflammation in Diarrhea-Predominant Patients with Irritable Bowel Syndrome
Digestive Diseases and Sciences (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.