Abstract
To improve the laundering durability of the silver functionalized antibacterial cotton fabrics, a radiation-induced coincident reduction and graft polymerization is reported herein where a pomegranate-shaped silver nanoparticle aggregations up to 500 nm can be formed due to the coordination forces between amino group and silver and the wrapping procedure originated from the coincident growth of the silver nanoparticles and polymer graft chains. This pomegranate-shaped silver NPAs functionalized cotton fabric exhibits outstanding antibacterial activities and also excellent laundering durability, where it can inactivate higher than 90% of both E. coli and S. aureus even after 50 accelerated laundering cycles, which is equivalent to 250 commercial or domestic laundering cycles.
Similar content being viewed by others
Introduction
Silver has been used as antibacterial material since ancient times. The study of antibacterial mechanisms have shown that free silver irons released from silver can effectively kill bacteria and is primarily responsible for silver's antibacterial property1,2,3,4,5.
Various forms of silver including zero-valent silver, silver oxide, ionic silver and silver-containing molecular complexes have been shown to be active against different bacterial strains. Recent work reveals that nanoscale forms of silver6,7,8,9 (and other antibacterial metals, copper10 and zinc oxide11 for example) are particularly effective at deactivating bacterial growth. Although the general antibacterial mechanism of nanoscale metal-based antimicrobial agents is largely unclear, its ultra-small size and super-large specific area has been proposed as a main rationale12,13,14,15,16.
Silver nanoparticles (NPs) are increasingly used to fabricate antimicrobial textiles17 for their broad-spectrum antibiotic property against different bacterial strains including common gram-negative bacteria, gram-positive bacteria18,19,20,21, even drug-resistant bacteria22,23,24, fungus, viruses and parasites25,26,27,28,29, e.g., Monkeypox Virus29 and HIV-127,28.
Laundering of such silver NPs-based textiles has already been identified as an important issue with concerns about increasing silver exposure in the environment and the general population30. Silver NPs can become released from textile surfaces into wastewater during laundering. The migration of silver NPs from the textile to human sweat can also increase dermal exposure, creating concerns about effects on human physiology17.
Various methods have been adopted for coating silver NPs on a textile surface, such as by generating active groups via plasma31,32 and UV irradiation33, or sol-gel processing34, in-situ reduction of the silver ions to metallic silver onto the fabrics35,36,37,38 and so on. However, conventional surface modification of textiles by silver NPs is not persistent, especially against laundering39,40. Thus, long-term antibacterial activity that resists home laundering is urgently needed41.
Some coordinating groups such as amino groups, carboxylic acid groups42 or hydrosulfide groups43, even the σ-bond formed with long alkyl chains44 have been further used to bind silver ions-containing silver NPs onto a textile surface. Antimicrobial Ag-loaded silk has been created through a one-step method of radiation-induced reduction and the results showed that Ag-loaded silk possessed some washing stability because the silver NPs interact with the amino acids on the surface of silk fibers via an electrostatic and coordination interaction45. The electrostatic and coordination interaction forces between ions, however, are insufficient against harsh laundering. It thus remains essential to introduce more powerful forces into the antibacterial system. Additionally, in consideration of concerns about increasing silver exposure in the environment and the general population, further attention needs to be paid to developing techniques that create lossless silver NPs during laundering. The strong binding of silver NPs onto the surface of the fabric not only provide the long-term effective antibacterial clothing which can keep us healthy while avoiding the abuse of antibiotics but also reduce the leakage of silver NPs into the environment with the waste water thus lessening the potential risks.
Here, we report a novel “one-step” radiation-induced coincident reduction of silver ions and graft polymerization of monomer containing amino group onto cotton fabric, to fabricate an antibacterial cotton fabric with a pomegranate-shaped structure of wrapped silver nanoparticle aggregations (NPAs) by polymer chains grafted onto the surface. The monomer, 2-aminoethyl methacrylate (AEMA), with a primary amine group that provides a coordination group and a carbon-carbon double bond that offers a grafting group was chosen in the experiment. The pomegranate-shaped polymer wrapped silver NPAs grown on the surface of the functionalized fabric allows an excellent laundering durability together with outstanding antibacterial activity, even after 50 accelerated laundering cycles, which is equivalent to 250 instances of home laundering. Meanwhile, the total loss of silver were below 10% after 50 accelerated laundering cycles, which means the product would be an environmental-friendly at the same time of protecting people's healthy.
Results
Wrapped silver NPAs formation
Cotton fabrics functionalized with pomegranate-shaped silver NPAs wrapped by poly(2-aminoethyl methacrylate) (PAEMA) graft chains were obtained by radiation-induced coincident reduction and graft polymerization. The cotton fabric samples were simply soaked in a solution of AgNO3 and an AEMA monomer with different concentrations and then irradiated by γ-ray up to an absorbed dose of 34 kGy under the protection of nitrogen gas. The sample was extracted by boiling milliQ water in a Soxhlet extractor for 24 hours to remove the unreacted AgNO3, residual monomer and homopolymers and then was vacuum dried for further measurements. The obtained sample was named as PAEMA-co-Ag cotton fabric in the following sections. The extraction of the product by hot water, is well accepted as the effective routine to remove not only the hydrophilic homopolymers46,47,48,49 but also the free silver ions35,36.
The PAEMA-co-Ag fabric looks slightly whitened as compared to pristine cotton fabric (Figure 1a). Hence, the success in graft polymerization can be proven by FT-IR spectroscopy study, where the new absorption band at 1,722 cm−1 in the spectrum of the PAEMA-co-Ag fabric can be attributed to an ester group in the PAEMA graft chains (Figure 1b). Due to the radiation-induced coincident reduction and graft polymerization, the weight increment ratio, which is called degree of grafting (DG), is composited by two sources, where DGPAEMA accounts for the PAEMA graft chains and DGAg accounts for the silver NPAs. DGAg is the weight ratio of silver NPAs attached on the fabric versus the pristine cotton fabric in percentage. Therefore, the silver contents can be re-written in mg/g unit by just multiply DGAg by 10, when the pristine cotton fabric is used as the substrate. Figure 1c shows the effect of the monomer concentration on the DGPAEMA and DGAg of PAEMA-co-Ag fabrics, where the Ag mass percentages were determined by microwave digestion followed by inductively coupled plasma-mass spectrometry (ICP-MS) and DGPAEMA were determined by subtracting DGAg from total DG. It was found that there was a tendency for the two DGs to work against each other with the PAEMA-co-Ag fabric, because of competition during the reduction reaction and graft polymerization, with both consuming the same amount of free radicals generated in the reaction system by γ-ray irradiation to the same total absorbed doses. Therefore, higher monomer concentration results in a higher reaction ratio of the graft polymerization which consumes more radicals and hence fewer radicals are left for the reduction reaction, resulting in a lower amount of generated silver NPAs.
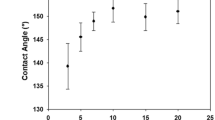
(a) Photos of the cotton fabric and the PAEMA-co-Ag fabric; (b) FT-IR ATR spectra of the cotton fabric and the PAEMA-co-Ag fabric; (c) Dependence of the DGPAEMA and DGAg of PAEMA-co-Ag fabrics on the AEMA monomer concentration under radiation-induced coincident reduction and graft polymerization; (d) Dependence of the DGPAEMA and DGAg of PAEMA-co-Ag fabrics on the Ag+ ion concentration under radiation-induced coincident reduction and graft polymerization; (e) Contact angle of the PAEMA-co-Ag fabric with DGAg = 0.54% and DGPAEMA = 14.6%.
Figure 1d shows the effect of the silver ion concentration on the DGPAEMA and DGAg of PAEMA-co-Ag fabrics with the monomer concentration was set at 10%. It was found that there was also a tendency for the two DGs to work against each other with, although the DGPAEMA seems less affected by the concentration of silver ions. DGAg is linearly increasing with the increase in log[Ag+]. The higher silver ion concentration, of cause, will raise DGAg. But over higher silver ion concentration could produce precipitation with the existence of chloride ions and also lead to the higher cost.
The contact angle (CA) results showed that the PAEMA-co-Ag fabric (DGAg = 0.54% and DGPAEMA = 14.6%) is superhydrophilic, with the water drops spreading out within 0.2 s (Figure 1e). The superhydrophilicity should be attributed to the hydrophilicity nature of the abundance in amino groups in the graft chains attached on the surface of the cotton fabric. The superhydrophilicity of PAEMA-co-Ag cotton fabric makes it more beneficial for the Ag NPs contact with solution such as our sweat and therefore the ionized silver could be release through the solution and kill bacteria effectively and continuously.
Additionally, XPS spectroscopy study was performed to confirm the existence and the chemical state of the elements on the surface of the fabrics. The successful introducing of silver NPs on the surface of PAEMA-co-Ag fabric by radiation is defined by the narrow scan spectra, where the new doublet peak of Ag(0) at 368 eV (3d5/2) and 374 eV (3d3/2) in the spectra of PAEMA-co-Ag fabric (Figure 2a). As contrast, there is no peaks attributed to Ag on the surface of the pristine cotton fabric and only PAEMA grafted cotton fabric. The coordination force of the amino group in the monomer with silver should account for the interaction of the PAEMA chains with the silver NPs, which can be defined by XPS N 1s spectroscopy study (Figure 2b), where the new peak of 407.7 eV attributed to the highly de-electronated state of N elements indicated the happening of the electron transfer between N and Ag elements, therefore, it means the strong interaction between the amino groups and the Ag NPs on the surface of the PAEMA-co-Ag fabric.
Figure 3a–3d shows the scanning electron microscopy (SEM) images of the pristine cotton fabric and PAEMA-co-Ag fabrics prepared under different monomer concentration. The radiation-induced simultaneous reduction and graft polymerization onto cotton fabric did not change the woven structure, which indicates good preservation of air-breathing properties that are important for clothing comfort.
SEM images of the cotton fabric (a,e) and the PAEMA-g-Ag fabric with DGAg = 0.82% and DGPAEMA = 10.9% (b,f), the PAEMA-g-Ag fabric with DGAg = 0.54% and DGPAEMA = 14.6%(c,g), the PAEMA-g-Ag fabric with DGAg = 0.16% and DGPAEMA = 21.1%(d,h).
The scale bars are 100 μm in (a,b,c,d) and 1 μm in (e,f,g,h).
Zoomed-in images show many nanoparticles attached to the surface of the fibers (Figure 3e–3h). Based on our knowledge46,47,48,49, there should be no particle formation on the surface of the fibers if only the methacrylate monomer was graft polymerized onto the cotton fabric under radiation. Therefore, these nanoparticles should be attributed to the silver NPs that were due to the reduction reaction. However, the size and numbers of silver NPs was found to not correspond with the DGAg of the PAEMA-co-Ag fabrics. At a DGAg of 0.82% and DGPAEMA of 10.9%, the size of the silver NPs were around 50 nm (Figure S1). When DGAg decreased to 0.54% and DGPAEMA increased to 14.6%, the size of the silver NPs suddenly increased to 500 nm (Figure S2). Further at a DGAg = 0.16% and DGPAEMA = 21.1%, the decrements left fewer and smaller nanoparticles to within 50 nm (Figure S3). Further, there were increasingly more wrinkled structures formed on the surface of the fibers with increasingly higher DGPAEMA, which can be attributed to aggregation of the grafted PAEMA chains. The abnormal size change in the silver NPs hinted that they are complex particles due to the interaction of the grafted polymer chains and the silver NPs, most possibly the pomegranate structure where the silver NPs are the seeds. This also could be partially proven by the color of the functionalized PAEMA-co-Ag fabric, which is almost unchanged when compared with pristine cotton fabric, indicating that the silver NPs were wrapped by PAEMA graft chains. If otherwise, the color of the functionalized fabric should be much darker if the silver NPs are exposed on the surface of the fibers, as demonstrated by the control samples.
To reveal the internal structure of the “pomegranates” grown on the surface of the fibers of PAEMA-co-Ag fabric (DGAg = 0.54% and DGPAEMA = 14.6%) (Figure 4a), high-resolution transmission electron microscope (TEM) images (Figure 4b and 4c) were taken, despite the difficulty in doing this due to the insulation nature of the fabric. From the images, the silver “seeds” were clearly found to be less than 10 nm in diameter with a lattice spacing of 0.25 nm and 0.236 nm belonging to the Ag 1/3{422} reflection and {111} facet50,51, which are well wrapped by the thick PAEMA “membrane” and “rind” (Figure 4d). The coordination force of the amino group in the monomer with silver should account for the interaction of the PAEMA chains with the silver NPs and the coincident growth of the silver NPs and PAEMA chains should account for the wrapping procedure. These two interactions are both important and resulted in the final pomegranate structure. The size of the “pomegranates” are determined by the reaction ratio of the reduction and graft polymerization. Seeding speed accompanied by a proper wrapping speed resulted in large fruits and explains why only a 10% monomer concentration at the fixed 0.01 mol/L AgNO3 concentration resulted in the wrapped silver NPAs pomegranate structure with sizes of up to hundreds of nanometers.
Control sample preparation
To demonstrate that the coordination force of the amino group with silver and the coincident reduction and graft polymerization are the two key processes for pomegranate-structure formation, we prepared two control samples. One control sample was prepared by radiation-induced coincident reduction and graft polymerization using a monomer, 1-allylurea, with a carbamido group instead of an amino group. The concentration of AgNO3 and the reaction conditions were exactly the same as that used in preparation of the PAEMA-co-Ag fabric. The product was named as PAU-co-Ag fabric. When the feed monomer concentrations were exactly the same as the PAEMA-co-Ag fabric with DGAg = 0.54% and DGPAEMA = 14.6%, the PAU-co-Ag fabric has a DGAg = 11.5% and DGPAU = 2.5% which should be attributed to the poor polymerization kinetic of AU.
The second control sample was prepared by radiation-induced graft polymerization followed by radiation-induced reduction. That is, the cotton fabric was grafted by AEMA and an intermediary product was named Cotton-g-PAEMA. The Cotton-g-PAEMA with DGPAEMA = 14.6% was then deposited with silver NPs by soaking in an AgNO3 solution and irradiated by γ-ray under nitrogen protection. The obtained sample was denoted as PAEMA-dep-Ag fabric and the corresponding DGAg is 0.12%.
After the functionalization, the color of the PAU-co-Ag and PAEMA-dep-Ag fabrics became chocolate brown and French gray, respectively (Figure S4). Due to the high amount of silver reduced onto the fabric, the SEM images of PAU-co-Ag fabric (Figure S5) show that there were many cubic- or cuboid-shaped silver NPs up to 400 nm in length deposited on the surface of the fiber and an absence of any round or wrapped structures. And in the SEM images of PAEMA-dep-Ag fabric (Figure S6), there were fewer and transformed cuboid-shape silver NPs up to 200 nm in the field of vision because of low amount of silver loading on the fabric. These results indicated the lack of coordination forces between amino groups and silver NPs together with incoincident reaction rate leading to giant silver NPs formation due to the trends to reduce surface energy. Also, the depositing of the silver NPs on the amino group's rich surface encountered with poor efficient and cannot result in a wrapped structure, which leads to fewer and free-standing silver NPs.
Anti-bacterial ability against E. coli and S. aureus
The antibacterial activity of pomegranate-shaped polymer wrapped in silver NPAs-based cotton fabrics was qualitatively evaluated and compared using gram-negative E. coli and gram-positive S. aureus bacteria cells according to American Association of Textile Chemists and Colorists (AATCC) Test method 100-2004 by TÜV SÜD PSB Product Testing (Shanghai) Co., Ltd. The detailed procedure is described in the Experiment section. After 24 hours of incubation at 37°C, a higher number of colony-forming units (CFU) appeared on pristine cotton and Cotton-g-PAEMA cotton fabrics that had no silver nanoparticles on their surfaces (Figure 5a–5d). In contrast, fewer E. coli and S. aureus cells were growing on the PAEMA-co-Ag fabric and fewer E. coli and S. aureus colonies were found (Figure 5e and 5f), implying that PAEMA-co-Ag fabric possess superior antibacterial activity. Subsequently, the loss of bacteria viability was calculated according to the inactivation efficiency test as detailed in the Experimental section. The results showed that PAEMA-co-Ag fabric possess excellent bactericidal activity and the inactivation activity was 96.5% for E. coli and 99.5% for S. aureus (Figure 5g), while cotton fabric and Cotton-g-PAEMA fabric were 0% for E. coli and S. aureus. PAEMA-co-Ag fabric was the excellent antibacterial material because of higher inactivation efficiency and lower Ag mass. Additionally, the inactivation efficiency of the PAEMA-co-Ag fabric for gram-positive bacteria was better than gram-negative, even though the gram-positive bacteria possessed more complicated cell wall structures.
(a–f) Images of the antibacterial activity test of the cotton fabric, cotton-g-PAEMA fabric and PAEMA-co-Ag fabric (DGAg = 0.54% and DGPAEMA = 14.6%) for E. coli and S. aureus; (g) The loss of viability for E. coli and S. aureus of the cotton fabric, cotton-g-PAEMA fabric and PAEMA-co-Ag fabric (DGAg = 0.54% and DGPAEMA = 14.6%).
Laundering durability
Laundering durability is an important and necessary factor for reuse of any modified fabric. A laundering durability test was carried out according to AATCC (American Association of Textile Chemists and Colorists) Test method 61− 2006, condition 2A52, tested with Without Optical Brightener (WOB) detergent (0.15%, w/w) and 50 stainless steel balls in warm water (Figure S7). One accelerated laundering cycle equals five home or commercial launderings. The inactivation efficiency of PAEMA-co-Ag fabric was measured after 5, 30 and 50 laundering cycles and the results were recorded. After 30 circles, the inactivated efficiencies of PAEMA-co-Ag fabric were still higher than 90% for both E. coli and S. aureus. After 50 wash cycles, the inactivation efficiency of PAEMA-co-Ag still remained higher than 90%, indicating that the sample possesses excellent laundering durability (Figure 6a).
(a) The loss of viability for E. coli and S. aureus of the PAEMA-co-Ag fabric (DGAg = 0.54% and DGPAEMA = 14.6%) after accelerated laundering test; SEM image of the pomegranate-shaped polymer wrapped in silver NPAs on the fiber before laundering test (b) and after 30 accelerated laundering cycles (c).
As for the control samples, after 30 laundering cycles, the inactivated efficiencies of PAEMA-dep-Ag was depressed to both types of bacteria (R = 14.1% to E. coli and R = 46.6% to S. aureus, Figure S8), which means it is unstable against laundering. Although the inactivated efficiency was around 80% to E. coli for PAU-co-Ag fabric after 30 laundering cycles (Figure S9), which was achieved by with an about 20 times higher amount of silver NPs loaded on the surface as compared with PAEMA-co-Ag fabric. Figure 6b and 6c shows the SEM images of the PAEMA-co-Ag fabric before and after 30 laundering tests and it is obvious that the pomegranates are retained well on the surface of the fibers of PAEMA-co-Ag fabric. But most of the silver NPs were washed off the surface of PAU-co-Ag (Figure S10) and PAEMA-dep-Ag fabric fibers (Figure S11). The results implied that PAEMA-co-Ag cotton could effectively reduce the outflow of silver NPs and therefore, showed excellent laundering durability. Based on inactivated efficiency after the laundering test, it is obvious that the PAEMA-co-Ag fabric is the most stable and efficient antibacterial functionalized cotton fabric, with the laundering stability originating from the polymer-wrapped silver NPAs pomegranate structure. Further, taking color changes into account, PAEMA-co-Ag fabric was the best choice in this present work.
In consideration of concerns about increasing silver exposure in the environment, silver ion concentrations in the washing liquid were characterized by the ICP-MS of the PAEMA-co-Ag fabric (Figure 7a). It is obvious that silver element loss is severe in the first 5 cycles, where the highest silver ion concentration is about 0.9 ppm in 150 mL of washing liquid and becomes steady after 20 cycles. Although the silver NPs in domestic effluent are concerned as expressed in the literature53,54,55, there is no national standard which gives the limitation of the silver NPs in waste water. For the easier understanding and operation, we measured the silver ion concentration instead here. The total silver ion of wash wastewater are limited to below 0.5 ppm according to the GB18466-2005, while the consumption of washing cloth are 15 L (water)/1 kg (cloth). The 0.9 ppm of silver ions in washing liquids was form 5 accelerated laundering cycles, which is equivalent to 25 instances of home laundering. Therefore, in real laundering, the silver ion concentration should be 0.18 ppm, which is much lower than emission standards of GB18466-2005, which show that the released silver from PAEMA-co-Ag cotton fabrics during washing should be acceptable.
(a) Ag ions concentration measured by ICP-MS in washing liquids of the PAEMA-co-Ag fabric (DGAg = 0.54% and DGPAEMA = 14.6%) in accelerated laundering test; (b) The cytotoxicity of washing liquid on human HaCaT cells. The cells were exposed to different concentrations (0%, 5%, 10%, 20%, v/v) of PAEMA-co-Ag washing liquid at 5th. accelerated laundering cycle and WOB detergent for 48 h.
In consideration of concerns about potential threats to humans from silver ions, the cytotoxicity of the diluted washing solution at the 5th cycle for PAEMA-co-Ag cotton fabrics was characterized using HaCaT cells. The washing solution and WOB detergent (0.15%, w/w) were diluted by Phosphate Saline Buffer (PBS) to a certain ratio and then used as the cultivation solution. An MTT assay based on mitochondrial succinic dehydrogenase was employed to detect the metabolic activity of HaCaT cells. As shown in Figure 7b, when the ratio is low, both the washing solution and WOB solution are almost harmless to the cell. But when the ratio increased to 20%, the WOB solution has extremely intense cell toxicity. Meanwhile the washing solution containing an extra 0.18 ppm of silver ions continued to show very low cell toxicity. From the cell morphology, it is obvious that HaCaT cells were slightly different with the blank ones with exposure to the washing solution, however, noticeable cell death occurred with exposure to the WOB solution (Figure S12). It is interesting that the silver ions in the washing solution depressed the cell toxicity of the detergent. And considering that this was an accelerated laundering test and the silver ion concentration is higher than home laundering, the released silver ions from PAEMA-co-Ag cotton fabrics during washing should be safe for human health.
Discussion
Pomegranate-shaped PAEMA wrapped silver NPAs functionalized cotton fabrics were prepared by radiation-induced coincident reduction and graft polymerization. The maximum size of the pomegranate-shaped silver NPAs can be up to 500 nm with a DGAg of 0.54% and DGPAEMA of 14.6%. The unique pomegranate-shaped formation can be attributed to the interaction of the PAEMA chains with the silver NPs that originate from coordination forces56 and the wrapping procedure originated from the coincident growth of the silver NPs and PAEMA chains, which cannot find on the surface of PAEMA-dep-Ag and PAU-co-Ag cotton fabric. This pomegranate-shaped silver NPAs functionalized cotton fabric exhibits outstanding antibacterial activities and also excellent laundering durability, where it can inactivate higher than 90% of both E. coli and S. aureus even after 50 accelerated laundering cycles (equivalent to 250 commercial or domestic laundering cycles), which are also superior to PAEMA-dep-Ag and PAU-co-Ag cotton fabric. This amazing performance and durability can be attributed to the superhydrophilic polymer wrapped silver NPAs where the silver NPAs are strongly covalent-bound to the cotton fibers, but the silver ions can be steadily released36. The simply radiation-induced coincident reaction is also promising for large-scale fabrication of antibacterial cotton fabrics thru pomegranate-shaped polymer wrapped silver NPAs.
Methods
PAEMA-co-Ag fabric preparation
The cotton fabric samples were put into irradiation tubes, then a 0.01 mol/L AgNO3 alcohol-water solution (1:9, vol.) with a defined monomer concentration was added to keep the samples immersed. The tubes were purged with nitrogen for 15 min and sealed and then irradiated with a 60Co γ-ray source at a constant dose rate of 2 kGy/h at room temperature for 17 h, until reaching a total absorbed dose of 34 kGy.
After the reaction, the samples were washed with large amounts of milliQ water and then the fabric was extracted for 24 h by hot milliQ water in a Soxhlet apparatus to remove residual monomer, homopolymers and unreacted ions. Finally, the samples were vacuum-dried to constant weight.
Determination of the degree of polymer grafting
DGAg denote the DG of silver NPAs on the cotton fabric, which measured by ICP-MS.
DGPAMEA was determined by gravimetric method, which was calculated by eq. (1)

where W0 and Wg denote the weights of the pristine and the grafted cotton fabric, respectively.
Inactivation efficiency test
An antibacterial activity test was carried out according to AATCC Test method 100–2004 by TÜV SÜD Products Testing (Shanghai) Co., Ltd. E. coli (ATCC 8739) or S. aureus (ATCC 6538) was vaccinated in LB (Luria Bertani broth, Lennox modification) liquid media for 24 hours at 37°C. The solution with bacterial cells was diluted to 1 ~ 2 c × 105/ml in 0.9% sodium chloride solution (NS). One ml of the final bacterial cell density of 1 ~ 2 c × 105/ml NS solution was evenly put onto different cotton fabrics (diameter = 4.8 cm, circle) which were sterilized in an autoclave at 105°C for 10 min and spread on the bottom of the jar. Then 100 mL neutralizing solution was added to the jar under vigorous agitation and incubated at 37°C and 90% RH for 24 h. 1 ml solution (at ‘0’ contact time) and 1 ml solution (after 24 h contact time) were diluted to 5 × 103/ml (2 × 103/ml, S. aureus) with 0.9% NS and then coated uniformly on three replicate LB agar plates (with 1.5% agar) per specimen solution (cotton as control group). These plates were incubated at 37°C for 24 h. The colony-forming units (CFU) spread on the agar plates were manually counted. The disk diffusion assay ha antibiotic controls. The reduction of bacteria named Loss of viability (%) was calculated with the following formulas:
Loss of viability (%) = (B-A)/B × 100% (1)
A: The number of bacteria recovered from the inoculated treated test specimen swatches in the jar incubated for 24 h.
B: The number of bacteria recovered from the inoculated treated test specimen swatches in the jar immediately after inoculation (at “0” contact time).
Laundering durability test
Laundering durability evaluation was carried out according to AATCC Test method 61− 2006, condition 2A. The samples were cut into 50 mm × 150 mm patches and washed in a rotating closed canister containing 150 mL aqueous solution of standard WOB detergent (0.15%, w/w) and 50 stainless steel balls in a thermostatically controlled water bath at 49°C, 40 ± 2 rpm. After being washed, the samples were washed using deionized water and vacuum dried for the inactivation efficiency test.
Cytotoxicity test
Human HaCaT cell lines (Cellbank, Chinese Academy of Sciences, China) were incubated in dulbecco's minimum essential medium (DMEM) medium (Gibco, USA) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS, Gibco, USA), penicillin (100 U/mL), streptomycin (100 μg/mL) in a humidified atmosphere containing 5% CO2 at 37°C.
Human HaCaT cell lines were seeded in a 24-well plate (5 × 104 cells/well) and grown overnight prior to the test. Then the cells were maintained in fresh media containing different concentrations of washing liquid. After 48 hours, the metabolism activities of cells were measured via MTT assay. Briefly, 50 μL of 5 mg/mL 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide solution (MTT, sigma, USA) was added to each well of the 24-well plate, followed by incubation at 37°C for 4 hours. Then cells were lysed with 10% acid sodium dodecyl sulfate solution (SDS, Sigma, USA). The absorbance of 200 μL cell lysis solution was measured at 570 nm by using a microplate reader (Bio-Rad 680, USA). The MTT assay was independently performed at least three times.
References
Lansdown, A. Silver. 2: Toxicity in mammals and how its products aid wound repair. J. Wound. Care. 11, 173 (2002).
Chernousova, S. & Epple, M. Silver as antibacterial agent: ion, nanoparticle and metal. Angew. Chem. Int. Ed. 52, 1636 (2013).
Kumar, R. & Münstedt, H. Silver ion release from antimicrobial polyamide/silver composites. Biomaterials. 26, 2081 (2005).
Marambio-Jones, C. & Hoek, E. M. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 12, 1531 (2010).
Glover, R. D., Miller, J. M. & Hutchison, J. E. Generation of metal nanoparticles from silver and copper objects: nanoparticle dynamics on surfaces and potential sources of nanoparticles in the environment. ACS Nano. 5, 8950 (2011).
Gondikas, A. P. et al. Cysteine-induced modifications of zero-valent silver nanomaterials: implications for particle surface chemistry, aggregation, dissolution and silver speciation. Environ. Sci.Technol. 46, 7037 (2012).
Gao, A. et al. The effects of titania nanotubes with embedded silver oxide nanoparticles on bacteria and osteoblasts. Biomaterials. 35, 4223 (2014).
Xiu, Z.-M. et al. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano letters. 12, 4271 (2012).
Lee, J. S., Suarez-Gonzalez, D. & Murphy, W. L. Tissue Engineering: Mineral Coatings for Temporally Controlled Delivery of Multiple Proteins. Adv. Mater. 23, 4278 (2011).
Grass, G., Rensing, C. & Solioz, M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 77, 1541 (2011).
Schwartz, V. B. et al. Antibacterial Surface Coatings from Zinc Oxide Nanoparticles Embedded in Poly (N-isopropylacrylamide) Hydrogel Surface Layers. Adv. Funct. Mater. 22, 2376 (2012).
Agarwal, A. et al. Polymeric multilayers that contain silver nanoparticles can be stamped onto biological tissues to provide antibacterial activity. Adv. Funct. Mater. 21, 1863 (2011).
Morones, J. R. et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 16, 2346 (2005).
Panáček, A. et al. Silver colloid nanoparticles: synthesis, characterization and their antibacterial activity. J. Phys. Chem. 110, 16248 (2006).
Rai, M. K. et al. The encapsulation technology in fruit plants—a review. Biotechnol. Adv. 27, 671 (2009).
Stark, W. J. Nanoparticles in biological systems. Angew. Chem. Int. Ed. 50, 1242 (2011).
Windler, L. et al. Release of titanium dioxide from textiles during washing. Environ. Sci. Technol. 46, 8181 (2012).
Bonde, S. et al. Murraya koenigii-mediated synthesis of silver nanoparticles and its activity against three human pathogenic bacteria. Nanosci. Methods. 1, 25 (2012).
Jung, W. K. et al. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 74, 2171 (2008).
Krishnaraj, C. et al. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloid. Surf. B. 76, 50 (2010).
Li, W.-R. et al. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 85, 1115 (2010).
Lv, M. et al. Long-Term Antimicrobial Effect of Silicon Nanowires Decorated with Silver Nanoparticles. Adv. Mater. 22, 5463 (2010).
Mühling, M. et al. An investigation into the effects of silver nanoparticles on antibiotic resistance of naturally occurring bacteria in an estuarine sediment. Mar. Environ. Res. 68, 278 (2009).
Rai, M., Deshmukh, S., Ingle, A. & Gade, A. Silver nanoparticles: the powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 112, 841 (2012).
George, C., Kuriakose, S., Prakashkumar, B. & Mathew, T. Synthesis, characterisation and antibacterial applications of water-soluble, silver nanoparticle-encapsulated β-cyclodextrin. Supramol. Chem. 22, 511 (2010).
Panáček, A. et al. Antifungal activity of silver nanoparticles against Candidaspp. Biomaterials. 30, 6333 (2009).
Elechiguerra, J. L. et al. Interaction of silver nanoparticles with HIV-1. J. Nanobiotechnol. 3, 1 (2005).
Lara, H. H., Ixtepan-Turrent, L., Garza-Treviño, E. N. & Rodriguez-Padilla, C. Research PVP-coated silver nanoparticles block the transmission of cell-free and cell-associated HIV-1 in human cervical culture. J. Nanobiotechnol. 13, 8 (2010).
Rogers, J. V. et al. A preliminary assessment of silver nanoparticle inhibition of monkeypox virus plaque formation. Nanoscale. Res. Lett. 3, 129 (2008).
Yu, S.-J., Yin, Y.-G. & Liu, J.-F. Silver nanoparticles in the environment. Environ. Sci.: Processes Impacts. 15, 78 (2013).
Vu, N. K. et al. Effect of Particle Size on Silver Nanoparticle Deposition onto Dielectric Barrier Discharge (DBD) Plasma Functionalized Polyamide Fabric. Plasma Process. Polym. 10, 285 (2013).
Rashidi, A. et al. Effect of plasma on the zeta potential of cotton fabrics. Plasma Sci. Technol. 15, 455 (2013).
Yuranova, T. et al. Antibacterial textiles prepared by RF-plasma and vacuum-UV mediated deposition of silver. J. Photoch. Photobio.A. 161, 27 (2003).
Mahltig, B., Haufe, H. & Böttcher, H. Functionalisation of textiles by inorganic sol–gel coatings. J. Mater. Chem. 15, 4385 (2005).
He, J., Kunitake, T. & Nakao, A. Facile in situ synthesis of noble metal nanoparticles in porous cellulose fibers. Chem. Mater. 15, 4401 (2003).
Potiyaraj, P., Kumlangdudsana, P. & Dubas, S. T. Synthesis of silver chloride nanocrystal on silk fibers. Mater. Letters. 61, 2464 (2007).
Perkas, N. et al. Ultrasound-assisted coating of nylon 6, 6 with silver nanoparticles and its antibacterial activity. J. Appli. Poly. Sci. 104, 1423 (2007).
Osório, I., Igreja, R., Franco, R. & Cortez, J. Incorporation of silver nanoparticles on textile materials by an aqueous procedure. Mater. Letters. 75, 200 (2012).
Impellitteri, C. A., Tolaymat, T. M. & Scheckel, K. G. The speciation of silver nanoparticles in antimicrobial fabric before and after exposure to a hypochlorite/detergent solution. J. Environ. Quality. 38, 1528 (2009).
Lorenz, C. et al. Characterization of silver release from commercially available functional (nano) textiles. Chemosphere. 89, 817 (2012).
Dastjerdi, R. & Montazer, M. A review on the application of inorganic nano-structured materials in the modification of textiles: focus on anti-microbial properties. Colloids. Surf. B. Biointerfaces. 79, 5 (2010).
Budama, L. et al. new strategy for producing antibacterial textile surfaces using silver nanoparticles. Chem. Eng. J. 228, 489 (2013).
Lin, J.-J. et al. S.-H. The cellular responses and antibacterial activities of silver nanoparticles stabilized by different polymers. Nanotechnology. 23, 065102 (2012).
Kennedy, D. C. et al. Carbon-bonded silver nanoparticles: alkyne-functionalized ligands for SERS imaging of mammalian cells. Chem. Commun. 47, 3156 (2011).
Chang, S., Kang, B., Dai, Y. & Chen, D. Synthesis of antimicrobial silver nanoparticles on silk fibers via γ-radiation. J. Appl. Polym. Sci. 112, 2511 (2009).
Deng, B. et al. Laundering Durability of Superhydrophobic Cotton Fabric. Adv. Mater. 22, 5473 (2010).
Cai, R. et al. Radiation induced graft polymerization of a fluorinated acrylate onto fabric. Radiat. Phys. Chem. 81, 1354 (2012).
Wu, J. X. et al. Self-healing of the superhydrophobicity by ironing for the abrasion durable superhydrophobic cotton fabrics. Sci. Rep. 3, 2951 (2013).
Yu, M. et al. Laundering Durability of Photocatalyzed Self-Cleaning Cotton Fabric with TiO2 Nanoparticles Covalently Immobilized. ACS Appl. Mater. Interf. 5, 3697 (2013).
Xie, J. P., Lee, J. Y., Wang, D. I. C. & Ting, Y. P. Silver Nanoplates: From Biological to Biomimetic Synthesis. ACS Nano. 1, 429 (2007).
Jiang, X. C., Zeng, Q. H. & Yu, A. B. A self-seeding coreduction method for shape control of silver nanoplates. Nanotechnology. 17, 4929 (2006).
AATCC Test Method 61–2006: Colorfastness to Laundering, Home and Commercial: Accelerated in AATCC TECHNICAL MANUAL, Vol. 82, American Association of Textile Chemists and Colorists, NC 27709, U.S.A. 2007.
Farkas et al. Characterization of the effluent from a nanosilver producing washing machine. Environ. Int. 37, 1057 (2011).
Wijnhoven et al. Nanotoxicology 3, 109 (2009).
Luoma, S. N. Silver Nanotechnologies and the Environment. The Project on Emerging Nanotechnologies Report 15 (2008).
Aymonier, C. et al. Hybrids of silver nanoparticles with amphiphilic hyperbranched macromolecules exhibiting antimicrobial properties. Chem. Commun. 24, 3018 (2002).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (11105210), the “Knowledge Innovation Program” of the Chinese Academy of Sciences (KJCX2-YW-N49).
Author information
Authors and Affiliations
Contributions
J.L. designed and supervised the project. H.L. and M.Y. performed the material preparation. H.L., M.L. and B.D. conducted the characterization. H.L., M.L., B.D., J.L., Q.H. and C.F. analysed the data. H.L., M.L., B.D. and J.L. wrote the manuscript. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
supporting information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Liu, H., Lv, M., Deng, B. et al. Laundering durable antibacterial cotton fabrics grafted with pomegranate-shaped polymer wrapped in silver nanoparticle aggregations. Sci Rep 4, 5920 (2014). https://doi.org/10.1038/srep05920
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep05920
This article is cited by
-
Ecofriendly fabrication of cobalt nanoparticles using Azadirachta indica (neem) for effective inhibition of Candida-like fungal infection in medicated nano-coated textile
Environmental Science and Pollution Research (2023)
-
A Variant Gel-Combustion Approach to Impregnate Nanostructured MgO Coating on Cotton Fibers for Antibacterial Textile Applications
Fibers and Polymers (2023)
-
Silver-based nanocomposite for fabricating high performance value-added cotton
Cellulose (2022)
-
Ampelopsis grossedentata Leaf Extract Induced Gold Nanoparticles as Wound Healing Dressing for Abdominal Wound Dehiscence in Nursing Care
Journal of Cluster Science (2022)
-
Development of antimicrobial, UV blocked and photocatalytic self-cleanable cotton fibers decorated with silver nanoparticles using silver carbamate and plasma activation
Cellulose (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.