Abstract
Adeno-associated virus (AAV) receptors range from heparan sulfate proteoglycan to sialic acid moieties present on cell surfaces. Abundance of the glycan profiles is greatly influenced by animal species, cell type and culture conditions. The objective of this study was to determine whether AAV serotypes' transduction efficiencies specifically in the equine monolayer culture model are an accurate representation of transduction efficiencies in tissue explants, a model more closely related to in vivo transduction. It was found that AAV 2 and 2.5 transduced cells more efficiently in explants than in monolayers. Through experiments involving assessing enzyme degradation of cell surface proteoglycans, this change could not be attributed to differences in the extra cellular matrix (ECM), but a similar change in AAV 5 transduction efficiency could be readily explained by differences in cell surface sialylated glycan. Unexpectedly it was found that in a small but diverse sample of horses evidence for serum neutralizing antibodies was only found to AAV 5. This suggests a unique relationship between this capsid and the equine host or an unresolved relationship between similar bovine AAV and the AAV 5 capsid immune response.
Similar content being viewed by others
Introduction
Gene therapy is currently being considered as a promising treatment for musculoskeletal diseases with considerable emphasis placed on arthritis1,2,3,4. Intra-articular gene therapy would target tissue using a vector that can infect articular cartilage and the synovial lining of the joint which contain the cell types chondrocytes and synoviocytes, respectively5,6. Osteoarthritis is characterized in the joint by tissue wear particles originating from the cartilage surface and increased inflammatory factors in the joint fluid7. Gene therapy has focused on supplementation of anabolic factors including insulin-like growth factor-I, transforming growth factor beta, bone morphogenetic protein 2 and inhibition of pro-inflammatory molecules using interleukin 4, soluble tumor necrosis factor receptor and interleukin-1 receptor antagonist protein which inhibits the pro-inflammatory interleukin-13,8,9,10,11,12. Treatment with these biological molecules requires long term and steady state dosing and both of these requirements are difficult to impossible using recombinant protein treatments but well suited for the gene therapy approach5,13. Various viral vectors have been studied for gene therapy use in the joint including herpes simplex virus, HIV based lentiviral vectors, adenovirus and adeno-associated virus (AAV)3,6,14,15. Adeno-associated virus as a therapeutic vector currently shows the most promise as it can genetically modify a variety of cells including non-dividing cells with minimal pathologic effects. As a result the gene of interest is expressed long term and tissue tropism is only limited by capsid serotype receptor accessibility2,16. The wide tissue tropism partly comes from the existence of different serotypes, at least 12 of which have been described with over 100 variants in different animal species17,18,19,20.
One challenge facing AAV as a gene therapy vector is the prevalence of neutralizing antibodies found in the patient population (>50% for AAV 2)21,22,23,24. The IgG antibodies from nonpathogenic AAV infections have been proven to induce a neutralizing effect on AAV in vitro22. These antibodies drastically decrease the transduction efficiency of the vector resulting in a treatment with limited to no success25. Due to the similarity between AAV capsid serotypes and the cross reactivity antibodies can have to the capsid, the most effective assay that determines transduction inhibition is a neutralization assay which directly tests whether the virus is not only bound by antibodies, but actually neutralized. These experiments have been performed in humans, but not in the horse to our knowledge19,21.
Cells in vitro are different in several ways from those in explants. A cell monolayer typically results in certain cell types based on adherence and growth conditions. Further cell passaging can induce dedifferentiation and phenotypic changes as shown in chondrocytes26. Exposure to cell surface and cell doubling is significantly different for monolayer versus explants, all critical variables that may influence vector transduction27. Many prior studies have been done with monolayer cell cultures due to the simplicity and ability to better control for variation in experiments. Because of the potential differences that may exist between transduction in monolayer and transduction of cells in situ, our goal was to test whether cell monolayers are a good approximation for transduction of tissue explant cultures.
The objective of this study was to determine whether transduction efficiencies in the monolayer culture model are an accurate representation of transduction efficiencies in tissue explants, a model more closely related to in vivo transduction. We hypothesized that there may be differences in transduction efficiencies due to the increased amount of extracellular matrix in explant tissues. Further, to maximize transduction efficiency in vivo we sought to investigate whether neutralizing antibodies existed in the joint fluid or the serum of the horse. We hypothesized that neutralizing antibodies would most likely exist to some of the AAV serotypes that have efficient transduction in equine synoviocytes and chondrocytes.
Results
Flow cytometry of vector transductions
Flow cytometry was performed on monolayer and tissue explant cultures to analyze the transduction efficiencies of AAV serotypes. The percentage of cells transduced and expressing GFP provided an indication of the proportion of the cells in the population that were permissive to an AAV transduction. In brief, cartilage explants, synovium explants, chondrocyte monolayers and synoviocyte monolayers were cultured and transduced with AAVs 1, 2, 2.5, 3, 4, 5 and 6 at a dose of 8,000 virus particles per cell (VPC). All AAVs were self-complimentary and encoded the gene for green fluorescent protein (GFP). This allowed for the flow cytometric determination of which AAV produced the highest percentage of cells expressing GFP and consequently which AAV was the most efficient at transducing the cell type/culture of interest. The results divided AAV serotypes into three classes based on preference for transducing explants or monolayers more efficiently: class I or those that transduced explants more efficiently than monolayer, class II or those that showed no difference between explant and monolayer transduction and class III or those that transduced monolayer more efficiently than explants. In cartilage derived cultures class I consisted of AAV 2 and 2.5; class II consisted of AAV 3 and 4; class III consisted of AAV 1, 5 and 6. In cultures derived from synovium, class I consisted of AAV 1, 2, 2.5 and 6. Class II consisted of AAV 3, 4 and 5 and there were no serotypes that could be categorized as class III (Figure 1). Flow cytometry revealed AAV2 and 2.5 to be the most efficient serotypes at transducing all tissue and culture types (Figure 2). Between the two, AAV 2 was consistently more efficient than 2.5, but this was not significant. AAV 3 was similar to 2 and 2.5 in all cultures except for synovial explants and AAV 5 in all but synovial and cartilage explants. AAV 1, 4 and 6 had varying amounts of statistical similarity to the other serotypes but consistently transduced cells the least efficiently of all serotypes. The difference in transduction efficiencies between cartilage explants and chondrocyte monolayers is not significant while averaging across all serotypes. This difference is significant however, when comparing synovial explants and synoviocyte monolayers.
Comparison of transduction efficiencies within the same tissue of origination but different culture types.
The graphs show flow cytometry analysis of the percent of cells transduced. Columns indicate mean percent and bars indicate standard error of the mean. Asterisks denote a significant difference between the explant and monolayer data in the specific AAV serotype tested. P-values < 0.05 are considered significant.
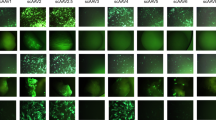
In a qualitative analysis of all the cultures (Figure 3 being a specific example) AAV 2 consistently appeared to have the best overall transduction efficiency. AAV 2.5 did not appear as efficient as the quantitative analysis showed. And finally AAV 1 and 4 often appeared to not transduce cells at all, while AAV 3, 5 and 6 had mixed results but usually presented transductions that were somewhat less efficient than AAV 2.
Fluorescence micrographs of the cell and culture types tested showing the presence or not of AAV transduction with the vectors tested.
Pictures are from a single animal twelve days after transduction. From top row to bottom row: cartilage explants, chondrocyte monolayers, synovial explants and synoviocyte monolayers.
Role of cell surface glycans
We next wished to compare the role of cell surface receptors with serotype specific transduction in explants versus monolayers. It was hypothesized that the differences in transduction efficiencies between explants and monolayers seen in Figure 1 could be attributed to varying concentrations of cell surface receptors for AAV. A previous report28 indicated that lack of heparan sulfate led to only a moderate decrease in AAV 2 transduction of cells. While the previous report examined the role of heparan sulfate, we also sought to determine the relationship of sialic acid (the other primary receptor specific to AAV 1, 4, 5 and 6)29,30. An experiment was performed where the primary cell surface receptors for AAV were enzymatically cleaved and transduction efficiencies compared to those of untreated controls. These experiments were carried out in cell monolayer and joint tissue explants at 1000 VPC in the presence of heparinase or neuraminidase. Enzyme treatment to remove cell surface receptors for AAV 2 included heparinase I and III, AAV 2.5 included both heparinase I and III and neuraminidase III and AAV 5 included neuraminidase III.
After enzyme treatment, there was no longer a statistical difference in transduction efficiencies between synovial explant and synoviocyte monolayer for AAV 2 and 2.5. The enzyme treatment also removed the statistical difference in transduction efficiencies between cartilage explant and chondrocyte monolayer for AAV 5 transduction. Of note, enzyme treatment did not remove the statistical difference in transduction efficiencies between cartilage explant and chondrocyte monolayer for AAV 2 and 2.5. It was interesting to note that AAV 2.5 is a rational design chimeric capsid containing 5 amino acids from AAV 1 spliced into the backbone of AAV 2 (ref. 31). While this capsid still utilizes heparan sulfate as the primary receptor, the inclusion of the additional five amino acids plays a significant but still unknown role in enhancing vector transduction in muscle. AAV 2.5 was the first example of a chimeric AAV vector used in a clinical trial31. The ability to mimic or partially mimic successful in vivo transduction with joint tissue explants offers the unique opportunity to exploit capsid library evolution that may confer a syntropic capsid for the joint superior to any of the several analyzed in this study.
Impact of neutralizing antibodies from equine serum and synovial fluid on serotype transduction
We next sought to understand the role of neutralizing antibodies. An experiment was performed to determine the concentrations of serum and synovial fluid that would prove to neutralize AAV transduction. In brief, serial dilutions of serum or synovial fluid were created from 1:12 to 1:200 with incomplete F12 media as the diluent. The 1:12 dilution would have the greatest concentration of serum or synovial fluid and consequently neutralizing antibodies (if present). These concentrations were then pre-incubated with AAVGFP particles for one hour prior to synoviocyte cell transduction. If neutralizing antibodies were found in any treatment, this would have prevented the AAVGFP particles from efficiently transducing the cells, resulting in fewer cells expressing the GFP transgene as quantified by flow cytometry measurement compared to a control transduction. Specifically, any neutralizing serum or synovial fluid treatment was recorded only if it inhibited at least half of the transduction compared to the untreated, positive control. Additionally, as an increase in concentration implies a commensurate increase in neutralizing factors, only the lowest concentration dilution of serum or synovial fluid was recorded as this would identify the minimum absolute amount of neutralizing antibodies required to effect a 50% change in transduction. This would additionally differentiate between the strength of neutralization against specific serotypes. For example, if a neutralizing concentration of 1:25 was demonstrated, the stronger concentration (1:12) would also cause neutralization. And for example, if neutralization at 1:200 was found against AAV1 and 1:25 against AAV2, this would imply a higher concentration and/or greater specificity of antibodies to AAV1 than to AAV2. It was found that no synovial fluid samples had neutralizing effects on AAV transduction (Table 1). Serum samples were only neutralizing to AAV 5 at the two highest concentrations tested and only to AAV 2 and 2.5 in one horse. Horses A and D were neutralizing to AAV 5 at a 1:12 dilution while horses B and C were neutralizing at a 1:25 dilution. Horse A was neutralizing to AAV 2 and 2.5 at only a 1:12 dilution.
Discussion
Using GFP transduction and neutralization assays we demonstrated differences between explant and monolayer transduction efficiencies in the joint tissues of the horse using a wide range of AAV serotypes. We observed three classes of AAV capsid transduction. The varying preference with which AAV serotypes transduce culture types was in agreement with findings by Mason et al.32 The serotype most effective at transduction overall was AAV 2. AAV 2.5, 3, 5 and 6 also were effective in varying culture conditions. This is in agreement with work done in monolayer cultures by Goodrich et al. that showed AAV 2, 3, 5 and 6 (AAV 2.5 was not included in the study) to be the most efficient serotypes tested33. In this study we extended the above analyses to the well-documented explant tissue model, previously shown to better represent in vivo joint tissue and surface glycans34,35,36. AAV 6 was found to be efficient in chondrocyte monolayers as in the previous study but less efficient in cartilage explants. Of note, AAV 2 and 2.5 were more effective in explants while AAV 5 and 6 were more effective in chondrocyte monolayers than in cartilage explants. This would predict that AAV 2 and 2.5 would perform better in vivo, all other variables being held constant. In fact, a recent study analyzing AAV 2 and 2.5 demonstrated persistent (80+ days) of increased transgene expression (400 ng/ml) supporting our explant studies37. The ability to mimic or partially mimic in vivo success with joint tissue explants offers the unique opportunity to exploit capsid library evolution that may offer syntropic capsids that are superior to the collection analyzed in this study.
To address the differences between explants and monolayers, an experiment was performed to enzymatically remove the cell surface receptors for AAV and determine if this equalized the AAV transduction efficiencies between the two. AAV 2 enzyme treatment included heparinase I and III, AAV 2.5 included both heparinase I and III and neuraminidase III and AAV 5 included neuraminidase III. AAV 2 uses the proteoglycan heparan sulfate as the major binding receptor and human fibroblast growth factor receptor 1 (FGFR1), hepatocyte growth factor receptor (HGFR) and the integrin αvβ5 as co-receptors38,39,40,41. It has been previously shown that AAV 2 transduction is not mediated by chondroitin sulfate moieties40. Perlecan, however, has been theorized to be a binding site for AAV based upon immunohistochemistry and a BLASTN search of AAV Rep recognition sequences within the human genome42,43. AAV 5 uses the sialylated glycan α-2,3-N-linked sialic acid for the major binding receptor44. AAV 2.5 is a chimera composed of AAV 2 with 5 amino acid substitutions from AAV 1 (which uses α-2,3- and α-2,6-N-linked sialic acid as major receptors). The primary receptor is still heparan sulfate and the role of the five amino acid substitutions is unknown20,30. This experiment revealed that a difference in AAV 5 transduction efficiency between cartilage explant and chondrocyte monolayer could be attributed to varying amounts of sialylated glycan. The difference in AAV 2 and 2.5 transduction efficiencies between cartilage explant and chondrocyte monolayer cannot be attributed to differences in heparin sulfate and heparan sulfate or sialylated glycan, respectively since removal of these receptors did not reduce transduction. Finally, the difference between synovial explant and synoviocyte monolayer transduction efficiencies for AAV 2 and 2.5 can be explained by differences in heparan sulfate and heparan sulfate or sialylated glycan, respectively. For experiments where changes in transduction efficiency between explants and monolayers are not explained by differences in primary cell surface receptors, there are several AAV secondary cell surface receptors20,30 that could be contributing to the variable efficiencies. The concentrations of enzymes used to remove cell surface glycans followed the procedures by Shen et al. and were nearly 1000 times those demonstrated to significantly reduce AAV transduction by Summerford et al.40,45 In several cases, the results of the AAV transductions did not match the efficiencies from Figure 4. This could be due to either using a lower titer of virus (which was necessary to observe the effects of the enzyme) of 1000 VPC (as opposed to 8000 VPC) or the fact that the cells were analyzed at ten days (as opposed to thirty). Although we expected a greater decrease in transduction in the positive control, this degree of drop in transduction has been reported in the past with this dose of viral particles per cell28. In contrast, Summerford and Samulski found an 80% decrease in rAAV transduction after heparinase treatment; however, this finding used a viral dose of 2 VPC. The significantly higher dose of AAV used in this study is hypothesized to have led to a smaller enzyme treatment mediated decrease in transduction due to increased binding by AAV to co-receptors HGFR, αVβ5 and FGFR138,39,41. Lower viral doses result in greater drops, however below 1000 viral particles per cell in joint tissues results in very low transduction efficiencies37. Regardless, it is of interest to determine if library select capsids with joint tropism utilize sialic acid or heparan sulfate on combination therapy.
Comparison of AAV transduction rates as percent of cells transduced in cultures treated with heparinases I and III (for AAV 2 and 2.5) and neuraminidase (for AAV 2.5 and 5).
Positive controls are denoted with dark fill and enzyme groups with light fill. Asterisks denote a significant difference between the enzyme treated and control group of the specific cell, culture and virus type tested. “-----” denotes a significant difference between enzymatically treated explant and monolayer cells of the same derived tissue within the specific serotype.
There were several horses that demonstrated various levels of AAV neutralization. All horses tested had no synovial fluid neutralization to any serotype of AAV but serum neutralization of varying degrees to AAV 5. Of note, bovine AAV shares capsid homology with AAV 546. The dilutions of serum that proved neutralizing in our study (1:12 and 1:25) could both be considered non-neutralizing according to an arbitrary cutoff of 1:50 that was used in the study by Boissier et al. and the 1:12 dilution could be considered non-neutralizing by the cutoff of 1:20 used in the study by Halbert et al21,24. The cutoff established by Halbert et al indicated dilutions of less than 1:20 can exhibit non-specific neutralization of AAV transduction, meaning AAV is neutralized by factors other than antibodies specific for the virus serotype. Horse A also revealed serum neutralization to AAV 2 and 2.5 but only at the highest concentration and neither of these neutralizations would be considered neutralizing by the previous cutoffs. There is known immune cross reactivity between serotypes so this test cannot prove whether a specific neutralized AAV serotype was responsible for a primary infection, if the neutralizing capability of the serum is indeed due to pre-existing antibodies. However, the experiment reveals that, in a clinical setting, it is favorable to use AAV 2, 2.5, 3 or 6 for gene therapy transduction in the horse. Others could theoretically be used if neutralization is tested before the gene therapy treatment. As neutralization was only seen in the serum, it would be most accurate for testing to use serum rather than synovial fluid for neutralization assays. This trend is in contrast to the work of Cottard et al. which demonstrated a strong correlation between serum and synovial fluid inhibition in humans22.
In conclusion, this study reveals that AAV transduction efficiency can differ between explants and monolayers. One of the contributing factors could be the increased amount of extracellular matrix found in the explant. This suggests that monolayer cultures could provide an adequate, relative model for testing transduction efficiency for AAV serotypes in vivo, but explants may offer a more accurate model. Additionally, we have shown there is a possibility of serum neutralization to AAV 5 in some horses. This should encourage clinicians to perform AAV 5 neutralization tests before administration of AAV gene therapy vectors to horses.
Methods
Transduction efficiencies
Tissues were harvested post mortem from four horses, aged two to five years old and euthanized for other reasons. Joints displayed no OA pathology. Synovium was aseptically excised from the inside of the fetlock joint capsule and cartilage from the patella. Similarly sized explants approximating 5 mm squares were cut from the larger pieces and kept in wells of 48 well plates with 500 μL Ham's F12 media (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA), 1× antibiotic antimycotic (HyClone, Logan, UT, USA) and 1N HEPES buffer (Invitrogen) changed every other day. The remaining synovium and cartilage pieces were diced and digested as previously described47. Cells were plated in appropriately sized tissue culture flasks at a density of 100,000/cm2 for synoviocytes and 200,000/cm2 for chondrocytes. Cells were grown for four days, then trypsinized and replated in 48 well plates at the aforementioned plating density. Two days following plating, cells and explants were transduced with scAAV-eGFP serotypes 1, 2, 2.5, 3, 4, 5 and 6 from the Gene Therapy Center Vector Core Facility (Chapel Hill, NC, USA). The vector scAAV-eGFP (pHpa-trs-SK) construct and cross packaging has been described previously48,49. Transduction was preceded by rinsing cells in PBS and then 250 μL Ham's F12 media was put into the wells. scAAV-eGFP was added to the wells at a concentration of 8000 virus particles per cell (VPC). The virus incubated with the cells for four hours at 37°C and 5% CO2 and was then aspirated and replaced by complete F12 media. The day of transduction was considered day zero. On days 4, 8, 12, 16 and 20, fluorescent microscopy pictures were taken of the cells and explants. The microscope used was an Olympus IX70 (Center Valley, PA, USA) with a filter cube with excitation at 495 nm and emission at 521 nm and at 100× magnification. Software used to capture images was QCapture by QImaging (Surrey, BC, Canada) and no post processing occurred. On day thirty, explants were individually digested and plated immediately into wells according to the prior digestion protocol. The explant cells adhered to the well for two days and then all wells were trypsinized. PBS with 0.2% FBS was added and the suspension analyzed by flow cytometry. Suspensions were run through a CyAn ADP Analyzer (Beckman Coulter, Brea, California, USA) and data analyzed with Summit 4.3 software (Dako, Carpinteria, CA, USA). The percent of cells transduced was the final data set analyzed. A mixed model ANOVA was used with horses being considered random variables. Statistics were considered significant with a p-value < 0.05.
Enzymatic desialylation and AAV transduction
Explants of cartilage and synovium and monolayers of chondrocytes and synoviocytes were isolated from a horse as described prior. Cultures were treated with heparinase I and III (Sigma-Aldrich #H2519 and #H8891, respectively) and/or neuraminidase III (Sigma-Aldrich #N7885) as described45. Briefly, cultures were treated with 3 U/ml heparinase I, 1.5 U/ml heparinase III, and/or 50 mU/ml neuraminidase in incomplete Ham's F12 media for two hours at 37°C and 5% CO2. Cultures were rinsed and scAAV-eGFP of 1000 VPC was added in incomplete Ham's F12 media and incubated for four hours at 37°C and 5% CO2. The lower level of 1000 VPC was shown to produce a more noticeable difference between enzyme treated and control samples as similarly found previously28. Finally, the virus suspension was removed and complete Ham's F12 media was added. At seven days post transduction the explants were digested and plated as previously described. On day ten the cells were analyzed with flow cytometry as described above. A fixed effects ANOVA was used for statistical analysis and significance was established at a p-value < 0.05.
AAV neutralizing antibodies
Synovial fluid and serum were collected post mortem from four horses euthanized for other reasons and independent to the horses prior. 293 cells were plated and grown in Dulbecco's modified Eagle's medium (DMEM, Invitrogen) supplemented as described above in 48-well plates to act as an indicator of AAV neutralization. Two days after plating, synovial fluid and serum dilutions of 1:12, 1:25, 1:50, 1:100 and 1:200 were created from the four animals with incomplete DMEM media. To the dilutions were added 8000 VPC of scAAV-eGFP serotypes 2, 2.5, 3, 5 and 6. The virus and serum or synovial fluid dilutions were incubated at 37°C and 5% CO2 for one hour. Following this incubation the mixtures were used in a four hour transduction of 293 cells. Post transduction the mixtures were replaced with complete DMEM. Six days post transduction the cells were analyzed with flow cytometry as described above. Neutralization was defined as a concentration of synovial fluid or serum that inhibited a transduction by at least 50% compared to the positive control (AAV treatment with no synovial fluid or serum). Only the neutralizing dilution with the weakest concentration of synovial fluid or serum was indicated for each combination of treatment type and serotype. Statistical analysis was performed using a fixed effects ANOVA and significance was established at a p-value < 0.05.
References
Ghivizzani, S. C. et al. Direct adenovirus-mediated gene transfer of interleukin 1 and tumor necrosis factor alpha soluble receptors to rabbit knees with experimental arthritis has local and distal anti-arthritic effects. Proc. Natl. Acad. Sci. U.S.A 95, 4613–4618 (1998).
Evans, C. H., Gouze, J. N., Gouze, E., Robbins, P. D. & Ghivizzani, S. C. Osteoarthritis gene therapy. Gene Ther. 11, 379–389, 10.1038/sj.gt.3302196 (2004).
Goodrich, L. R. et al. Direct adenovirus-mediated IGF-I gene transduction of synovium induces persisting synovial fluid IGF-I ligand elevations. Gene Ther. 13, 1253–1262, 10.1038/sj.gt.3302757 (2006).
Goodrich, L. R., Hidaka, C., Robbins, P. D., Evans, C. H. & Nixon, A. J. Genetic modification of chondrocytes with insulin-like growth factor-1 enhances cartilage healing in an equine model. J. Bone Joint Surg. Br. 89B, 672–685, 10.1302/0301-620x.89b5.18343 (2007).
Watanabe, S. et al. Adeno-associated virus mediates long-term gene transfer and delivery of chondroprotective IL-4 to murine synovium. Mol. Ther. 2, 147–+ (2000).
Madry, H., Cucchiarini, M., Terwilliger, E. F. & Trippel, S. B. Recombinant adeno-associated virus vectors efficiently and persistently transduce chondrocytes in normal and osteoarthritic human articular cartilage. Hum. Gene Ther. 14, 393–402 (2003).
Goldring, S. R. & Goldring, M. B. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin. Orthop. Relat. Res. S27–S36, 10.1097/01.blo.0000144854.66565.8f (2004).
Frisbie, D. D., Ghivizzani, S. C., Robbins, P. D., Evans, C. H. & McIlwraith, C. W. Treatment of experimental equine osteoarthritis by in vivo delivery of the equine interleukin-1 receptor antagonist gene. Gene Ther. 9, 12–20 (2002).
Glansbeek, H. L., van Beuningen, H. M., Vitters, E. L., van der Kraan, P. M. & van den Berg, W. B. Stimulation of articular cartilage repair in established arthritis by local administration of transforming growth factor-beta into murine knee joints. Lab. Invest. 78, 133–142 (1998).
Glansbeek, H. L. et al. Bone morphogenetic protein 2 stimulates articular cartilage proteoglycan synthesis in vivo but does not counteract interleukin-1 alpha effects on proteoglycan synthesis and content. Arthritis Rheum. 40, 1020–1028, 10.1002/art.1780400605 (1997).
Lubberts, E. et al. IL-4 gene therapy for collagen arthritis suppresses synovial IL-17 and osteoprotegerin ligand and prevents bone erosion. J. Clin. Invest. 105, 1697–1710 (2000).
Kim, S. H. et al. Ex Vivo Gene Delivery of IL-1Ra and Soluble TNF Receptor Confers a Distal Synergistic Therapeutic Effect in Antigen-Induced Arthritis. Mol. Ther. 6, 591–600 (2002).
Evans, C. H., Ghivizzani, S. C. & Robbins, P. D. Getting arthritis gene therapy into the clinic. Nat. Rev. Rheumatol. 7, 244–249, 10.1038/nrrheum.2010.193 (2011).
Oligino, T. et al. Intra-articular delivery of a herpes simplex virus IL-1Ra gene vector reduces inflammation in a rabbit model of arthritis. Gene Ther. 6, 1713–1720 (1999).
Gouze, E. et al. In vivo gene delivery to synovium by lentiviral vectors. Mol. Ther. 5, 397–404, 10.1006/mthe.2002.0562 (2002).
Ulrich-Vinther, M. Gene therapy methods in bone and joint disorders - Evaluation of the adeno-associated virus vector in experimental models of articular cartilage disorders, periprosthetic osteolysis and bone healing. Acta Orthop. 78, 5–64 (2007).
Choi, V. W., McCarty, D. M. & Samulski, R. J. AAV hybrid serotypes: Improved vectors for gene delivery. Curr. Gene Ther. 5, 299–310 (2005).
Schmidt, M. et al. Adeno-associated virus Type 12 (AAV12): A novel AAV serotype with sialic acid- and heparan sulfate proteoglycan-independent transduction activity. J. Virol. 82, 1399–1406, 10.1128/jvi.02012-07 (2008).
Gao, G. P., Vandenberghe, L. H. & Wilson, J. M. New recombinant serotypes of AAV vectors. Curr. Gene Ther. 5, 285–297 (2005).
Wu, Z. J., Asokan, A. & Samulski, R. J. Adeno-associated virus serotypes: Vector toolkit for human gene therapy. Mol. Ther. 14, 316–327, 10.1016/j.ymthe.2006.05.009 (2006).
Halbert, C. L. et al. Prevalence of Neutralizing Antibodies Against Adeno-Associated Virus (AAV) Types 2, 5 and 6 in Cystic Fibrosis and Normal Populations: Implications for Gene Therapy Using AAV Vectors. Hum. Gene Ther. 17, 440–447, 10.1089/hum.2006.17.440 (2006).
Cottard, V. et al. Immune response against gene therapy vectors: Influence of synovial fluid on adeno-associated virus mediated gene transfer to chondrocytes. J. Clin. Immunol. 24, 162–169 (2004).
Boutin, S. et al. Prevalence of Serum IgG and Neutralizing Factors Against Adeno-Associated Virus (AAV) Types 1, 2, 5, 6, 8 and 9 in the Healthy Population: Implications for Gene Therapy Using AAV Vectors. Hum. Gene Ther. 21, 704–712, 10.1089/hum.2009.182 (2010).
Boissier, M. C. et al. Synoviocyte infection with adeno-associated virus (AAV) is neutralized by human synovial fluid from arthritis patients and depends on AAV serotype. Hum. Gene Ther. 18, 525–535, 10.1089/hum.2006.174 (2007).
Scallan, C. D. et al. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood 107, 1810–1817, 10.1182/blood-2005-08-3229 (2006).
Lin, Z. et al. Gene expression profiles of human chondrocytes during passaged monolayer cultivation. J. Orthop. Res. 26, 1230–1237, 10.1002/jor.20523 (2008).
Alexander, I. E., Russell, D. W., Spence, A. M. & Miller, A. D. Effects of gamma irradiation on the transduction of dividing and nondividing cells in brain and muscle of rats by adeno-associated virus vectors. Hum. Gene Ther. 7, 841–850 (1996).
Boyle, M. et al. Membrane-associated heparan sulfate is not required for rAAV-2 infection of human respiratory epithelia. Virology 3, 29 (2006).
Kaludov, N., Brown, K. E., Walters, R. W., Zabner, J. & Chiorini, J. A. Adeno-Associated Virus Serotype 4 (AAV4) and AAV5 Both Require Sialic Acid Binding for Hemagglutination and Efficient Transduction but Differ in Sialic Acid Linkage Specificity. J. Virol. 75, 6884–6893, 10.1128/jvi.75.15.6884-6893.2001 (2001).
Wu, Z. J., Miller, E., Agbandje-McKenna, M. & Samulski, R. J. alpha 2,3 and alpha 2,6 N-linked sialic acids facilitate efficient binding and transduction by adeno-associated virus types 1 and 6. J. Virol. 80, 9093–9103, 10.1128/jvi.00895-06 (2006).
Bowles, D. E. et al. Phase 1 Gene Therapy for Duchenne Muscular Dystrophy Using a Translational Optimized AAV Vector. Mol. Ther. 20, 443–455, http://www.nature.com/mt/journal/v20/n2/suppinfo/mt2011237s1.html (2012).
Mason, J. B., Vandenberghe, L. H., Xiao, R., Wilson, J. M. & Richardson, D. W. Influence of serotype, cell type, tissue composition and time after inoculation on gene expression in recombinant adeno-associated viral vector–transduced equine joint tissues. Am. J. Vet. Res. 73, 1178–1185, 10.2460/ajvr.73.8.1178 (2012).
Goodrich, L. R., Choi, V. W., Carbone, B. A. D., McIlwraith, C. W. & Samulski, R. J. Ex Vivo Serotype-Specific Transduction of Equine Joint Tissue by Self-Complementary Adeno-Associated Viral Vectors. Hum. Gene Ther. 20, 1697–1702, 10.1089/hum.2009.030 (2009).
Collier, S. & Ghosh, P. Effects of transforming growth factor beta on proteoglycan synthesis by cell and explant cultures derived from the knee joint meniscus. Osteoarthr. Cartilage 3, 127–138, 10.1016/S1063-4584(05)80045-7 (1995).
Flannery, C. R., Little, C. B., Caterson, B. & Hughes, C. E. Effects of culture conditions and exposure to catabolic stimulators (IL-1 and retinoic acid) on the expression of matrix metalloproteinases (MMPs) and disintegrin metalloproteinases (ADAMs) by articular cartilage chondrocytes. Matrix Biol. 18, 225–237, 10.1016/S0945-053X(99)00024-4 (1999).
Collier, S. & Ghosh, P. Comparison of the effects of non-steroidal anti-inflammatory drugs (NSAIDs) on proteoglycan synthesis by articular cartilage explant and chondrocyte monolayer cultures. Biochem. Pharmacol. 41, 1375–1384, 10.1016/0006-2952(91)90111-H (1991).
Goodrich, L. R. et al. Optimization of scAAVIL-1ra In Vitro and In Vivo to Deliver High Levels of Therapeutic Protein for Treatment of Osteoarthritis. Mol. Ther. Nucleic Acids 2, e70, 10.1038/mtna.2012.61 (2013).
Kashiwakura, Y. et al. Hepatocyte Growth Factor Receptor Is a Coreceptor for Adeno-Associated Virus Type 2 Infection. J. Virol. 79, 609–614, 10.1128/jvi.79.1.609-614.2005 (2005).
Summerford, C., Bartlett, J. S. & Samulski, R. J. alpha V beta 5 integrin: a co-receptor for adeno-associated virus type 2 infection. Nat. Med. 5, 78–82 (1999).
Summerford, C. & Samulski, R. J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J. Virol. 72, 1438–1445 (1998).
Qing, K. et al. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat. Med. 5, 71–77 (1999).
Konishi, M., Kawamoto, K., Izumikawa, M., Kuriyama, H. & Yamashita, T. Gene transfer into guinea pig cochlea using adeno-associated virus vectors. J. Gene Med. 10, 610–618, 10.1002/jgm.1189 (2008).
Wonderling, R. S. & Owens, R. A. Binding sites for adeno-associated virus rep proteins within the human genome. J. Virol. 71, 2528–2534 (1997).
Walters, R. W. et al. Binding of Adeno-associated Virus Type 5 to 2,3-Linked Sialic Acid Is Required for Gene Transfer. J. Biol. Chem. 276, 20610–20616, 10.1074/jbc.M101559200 (2001).
Shen, S., Bryant, K. D., Brown, S. M., Randell, S. H. & Asokan, A. Terminal N-Linked Galactose Is the Primary Receptor for Adeno-associated Virus 9. J. Biol. Chem. 286, 13532–13540, 10.1074/jbc.M110.210922 (2011).
Johnson, F. B., Fenn, L. B., Owens, T. J., Faucheux, L. J. & Blackburn, S. D. Attachment of bovine parvovirus to sialic acids on bovine cell membranes. J. Gen. Virol. 85, 2199–2207, 10.1099/vir.0.79899-0 (2004).
Nixon, A. J., Lust, G. & Verniersinger, M. Isolation, propogation and cryopreservation of equine articular chondrocytes. Am. J. Vet. Res. 53, 2364–2370 (1992).
McCarty, D. M., Monahan, P. E. & Samulski, R. J. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 8, 1248–1254 (2001).
Rabinowitz, J. E. et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J. Virol. 76, 791–801, 10.1128/jvi.76.2.791-801.2002 (2002).
Acknowledgements
This work was conducted with support from NIH grant K08AR054903 (Goodrich, McIlwraith, Samulski), 1R01DK084033 (Samulski) 2-R01-AI072176-06A1 (Samulski) P01 HL112761-01A1 (Samulski) and a grant from the Colorado State University College Research Council (Hemphil, Goodrich). We would like to acknowledge the Gene Therapy Center Vector Core at the University of North Carolina at Chapel Hill for providing the scAAV-eGFP vectors of all serotypes.
Author information
Authors and Affiliations
Contributions
D.D.H. wrote the main manuscript. D.D.H., C.W.M., R.J.S. and L.R.G. reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Hemphill, D., McIlwraith, C., Samulski, R. et al. Adeno-Associated Viral Vectors Show Serotype Specific Transduction of Equine Joint Tissue Explants and Cultured Monolayers. Sci Rep 4, 5861 (2014). https://doi.org/10.1038/srep05861
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep05861
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.