Abstract
In this work, a rapid (within 4–5 h), sensitive and visible new method for assessing botanic origin is developed by combining loop-mediated isothermal amplification with cationic conjugated polymers. The two Chinese medicinal materials (Jin-Yin-Hua and Shan-Yin-Hua) with similar morphology and chemical composition were clearly distinguished by gene SNP genotyping assays. The identification of plant species in Patented Chinese drugs containing Lonicera buds is successfully performed using this detection system. The method is also robust enough to be used in high-throughput screening. This new method is very helpful to identify herbal materials and is beneficial for detecting safety and quality of botanic products.
Similar content being viewed by others
Introduction
An increasing number of functional foods, medicines and cosmetics containing botanical components have been gaining popularity worldwide. However, fresh and dried organs of some plants exhibit similar morphology, making them difficult to distinguish and creating safety issues with their use. It can be even more difficult to know with certainty the botanical origin of processed powders and other finished products. For example, components of two Chinese medicines, Jin-Yin-Hua and Shan-Yin-Hua, belong to the genus Lonicera. Jin-Yin-Hua includes Lonicera japonica buds, while Shan-Yin-Hua includes buds of L. hypoglauca, L. macranthoides, L. confusa and L. fulvotomentosa1.
Because it is a highly important antiviral and antibacterial herbal medicine, the price of Jin-Yin-Hua is much higher than that of Shan-Yin-Hua. The two materials have similar morphology and chemical composition but different medicinal usages; thus, correct identification of plant species is extremely important. Traditional methods for identifying plant components include high-performance liquid chromatography (HPLC)2, gas chromatograph-mass spectrometry (GC-MS)3 and thin-layer chromatography (TLC)4. However, very slight differences in chemical compounds have been identified in relative species and chemical composition is also affected by environmental conditions, plant development and processing5.
To improve the precision of identifying components of interest, genetic techniques, such as polymerase chain reaction (PCR) for genotyping single-nucleotide polymorphisms (SNP)6,7,8,9,10 have been used to differentiate plants at the species level11,12,13,14,15. Using sequencing analysis, we previously identified a C/A SNP site in trnL-trnF between Jin-Yin-Hua and Shan-Yin-Hua and we developed an allele-specific PCR (AS-PCR) system for identifying the two medicinal materials16. Since only trace amounts of botanical materials are included in processed products, a simple, rapid and sensitive method for identifying these materials is required to reach the extent of visual resolution.
In recent years, we have taken advantage of the excellent light-harvesting ability of conjugated polymers to enhance the sensitivity of DNA detection17,18. Electrostatic complexes of cationic conjugated polymers with DNA have been designed for homogeneous, sensitive and simple fluorescence assays for SNP genotyping19,20,21,22. Furthermore, loop-mediated isothermal amplification (LAMP), which operates at a consistent temperature and only use a heating equipment, has been described as an easy and rapid tool for amplification of target DNA23,24,25,26. Here, we demonstrate a simple, rapid, sensitive and high-throughput system that combines LAMP with cationic conjugated polymers for screening botanical origin in multi-color and one-tube SNP genotyping assays. The identification of plant species in patented Chinese drugs containing Lonicera buds is successfully performed using this detection system.
Results
Figure 1 illustrates the principles for screening botanical origin. A sequence in LoniceratrnL-trnF that contains a polymorphic site is used as a DNA target in which nucleotide C in the wild type (Jin-Yin-Hua) is replaced by nucleotide A in the mutant target (Shan-Yin-Hua). Three SNP genotypes are thus possible: homozygous C (Jin-Yin-Hua), homozygous A (Shan-Yin-Hua) and heterozygous C/A (mixture). Two pairs of LAMP primers (F3/B3, FIP/BIP) and Bst DNA polymerase were used for amplification of the target DNA. A water-soluble cationic conjugated polymer, poly[(9,9-bis(6′-N,N,N-triethylammonium) hexyl) fluorenylene phenylene] (PFP), was used as a donor in fluorescence resonance energy transfer (FRET) experiments. Fluorescein-labeled ddUTP (ddUTP-Fl) and cyanine 3 (Cy3)-labeled deoxyguanosine triphosphate (dGTP) were chosen as acceptors. PFP acts as the donor for fluorescein and Cy3; fluorescein acts as the acceptor for PFP and the donor for Cy3 to satisfy the overlap integral requirement for FRET. The primer is complementary to the wild target and to the mutant target immediately upstream of the polymorphic site. Taq DNA polymerase, ddUTP-Fl and dGTP-Cy3 were used for primer-extension reactions. For the homozygous C genotype, only dGTP-Cy3 was incorporated into the primer; upon addition of PFP, strong electrostatic interactions caused attraction between negatively charged DNA and cationic PFP and efficient FRET from PFP to Cy3 occurred. For the homozygous A genotype, only ddUTP-Fl was incorporated into the primer and efficient FRET occurred from PFP to Fl. For the heterozygous C/A genotype, ddUTP-Fl and dGTP-Cy3 were incorporated into the primers for the mutant and wild targets, respectively. In this case, upon exciting PFP at 380 nm, multi-step FRET occurred.
Dections of the origin of Jin-Yin-Hua and Shan-Yin-Hua was performed using LAMP and CCP-based method; the three genotypes could be clearly distinguished by different colors. The maximum emission of PFP in buffer solution appeared at approximately 425 nm and no emission of fluorescein at 530 nm or of Cy3 at 574 nm was observed (Figure 2a and 2b). For homozygous A (Shan-Yin-Hua), efficient FRET from PFP to fluorescein led to a significant quenching of PFP emission at 425 nm and appearance of the fluorescein emission at 530 nm (Figure 2c). Images of the extension products with PFP dropped on a glass slide under 300 nm UV light with transmission mode are presented in Figure 2d. A band-pass optical filter was used to filter the emission of the excitation source and PFP. The solution exhibited a green emission color (Figure 2a and 2d). Emission of Cy3 was observed for homozygous C (Jin-Yin-Hua) and the solution emitted a purplish red color (Figure 2d). Emission of both fluorescein and Cy3 was observed for heterozygous C/A (mixture) and this solution was pinky-white in color (Figure 2b and 2d).
(a) Emission spectra of PFP in the presence of Jin-Yin-Hua and Shan-Yin-Hua. (b) Emission spectra of PFP in mixture. (c) Integrated emission intensities. (d) A photograph of fluorescence pattern on a microplate corresponding to Jin-Yin-Hua, Shan-Yin-Hua and their mixture. The solutions contain PFP and extension products of homozygous C (Jin-Yin-Hua), heterozygous C/A (Mixture) and homozygous A (Shan-Yin-Hua). A no-template control (NTC) was used as the blank.
Thre shold values used to estimate botanical origin were plotted according to Equation (1):

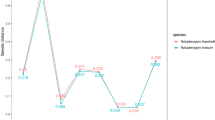
Where RCFRET represents the relative change of FRET ratio; and “FRET0” and “FRET” are the initial and target-added ratios of I574 nm/I425 nm or I530 nm/I425 nm, respectively. Threshold values of Jin-Yin-Hua were determined as RCFRET (I530 nm/I425 nm) ≤ 2.3 and RCFRET (I574 nm/I425 nm) ≥ 3.5 (Figure 3a and 3b). All (192/192) of Shan-Yin-Hua and 92.7% (47/48) of the mixture were separated from Jin-Yin-Hua.
(a) Threshold values of Jin-Yin-Hua (96/96) and Shan-Yin-Hua (192/192). (b) Threshold values of Jin-Yin-Hua (96/96) and mixture (47/48) with equal DNA concentration of Jin-Yin-Hua and Shan-Yin-Hua. Emission spectra (c) and threshold values (d) from solutions containing PFP and extension products in the presence of varying concentrations of heterozygous C/A (mixture with equal DNA concentration of Jin-Yin-Hua and Shan-Yin-Hua). No-template control (NTC) was used as the blank.
To investigate the dynamic range of the target concentration, the emission spectra of a series of extension products containing various concentrations of heterozygous C/A (equal DNA concentration of Jin-Yin-Hua and Shan-Yin-Hua) were measured with excitation wavelength 380 nm after adding PFP (Figure 3c). The dynamic range at which the mixed DNA was separate from the DNA of Jin-Yin-Hua was 0.05 to 50 ng μl−1(Figure 3d). To further investigate the dynamic range of the mixture, the emission spectra of a series of extension products containing various percentages of Jin-Yin-Hua and Shan-Yin-Hua were measured at excitation wavelength 380 nm after adding PFP (Figure 4a, Figure S4). The limit of detection (LOD) of this method was obtained from seven independent measurements using Equation (2):

Where S0 is the standard deviation of the background and S is the sensitivity. The relative standard deviation for seven measurements of the blank was 6.2% and a LOD for Shan-Yin-Hua of 0.84% was achieved with the proposed method. Thus, this method afforded high sensitivity for an SNP genotyping assay.
(a) Threshold values of mixture containing various ratios of Jin-Yin-Hua and Shan-Yin-Hua, including 100:0 (only Jin-Yin-Hua), 95:5 (5% of Shan-Yin-Hua), 90:10 (10% of Shan-Yin-Hua), 80:20 (20% of Shan-Yin-Hua), 70:30 (30% of Shan-Yin-Hua), 60:40 (40% of Shan-Yin-Hua), 50:50 (50% of Shan-Yin-Hua) and 0:100 (only Shan-Yin-Hua). (b) Threshold values of botanic origin in Patented Chinese drugs.
We selected twelve patented Chinese drugs (Qin Re An Chuang Wan, Lin Yan Jie Du Ke Li, Jian Nao Bu Shen Wan, Jin Sang San Jie Wan, Niu Huang Qing Gong Wan, Xiao Er Yan Bian Ke Li, Gan Mao Zhi Ke Ke Li, Lian Qiao Bai Du Wan, Zhi Zi Jin Hua Wan, Qing Guo Wan, Fu Fang Zhen Zhu An Chuang Pian and Xiao Er Gan Mao Ning Tang Jiang) that contained Lonicera buds and screened their botanical origin using this detection system. The RCFRET (I530 nm/I425 nm) and RCFRET (I574 nm/I425 nm) for Qin Re An Chuang Wan, Lin Yan Jie Du Ke Li and Jian Nao Bu Shen Wan fell within the threshold values of those of Jin-Yin-Hua (Figure 4b), suggesting that the plant materials shared an origin in L. japonica (Table 1).
Discussion
Combining loop-mediated isothermal amplification with cationic conjugated polymers, we have performed a rapid (4–5 h), sensitive and visible new method for assessing botanical origin from DNA extraction, LAMP amplification and single-base extension to detection of botanical origin. Water-soluble cationic conjugated polymer PFP, was used as a donor in fluorescence resonance energy transfer (FRET) experiments. Fluorescein-labeled ddUTP and Cy3-labeled dGTP were chosen as acceptors. PFP acts as the donor for fluorescein and Cy3; fluorescein acts as the acceptor for PFP and the donor for Cy3 to satisfy the overlap integral requirement for FRET. By triggering a shift in emission color or a change in the emission intensity of PFP, fluorescein, or Cy3, it was possible to assay the three SNP genotypes in one extension reaction. Thus, Jin-Yin-Hua, Shan-Yin-Hua and their mixture could be simultaneously differentiated and maximum visual resolution could be achieved by using this mathod.
Two Chinese medicinal materials (Jin-Yin-Hua and Shan-Yin-Hua) with similar morphology and chemical composition were clearly distinguished by SNP genotyping assays. The identification of plant species in patented Chinese drugs containing Lonicera buds was performed successfully using this method, which is robust enough to be used in high-throughput screening. Further, no complicated instrumentation or expensive reagents are utilized in the assay, making the method cost effective. This new method will be advantageous for identifying herbal materials and for determining the safety and quality of botanical products.
Methods
Allele-specific PCR detection of samples at SNP site C/A
A C/A SNP site in trnL-trnF between Jin-Yin-Hua and Shan-Yin-Hua by sequencing analysis (Supporting Information Figure S1). Genomic DNA was isolated from samples (25 mg each) using the modified CTAB method27. Total genomic DNA was used in the allele-specific PCR according to the protocol established by Jiang et al16. PCR was performed by adding 2.5 μl 10× rTaq Taq buffer, 2 μl 10 mM dNTPs, 0.5 U rTaq (Takara, China), 0.2 pmol of each forward trnL.F and reverse primer trnL.R (Supporting Information Table S3) and 10 ng DNA per reaction volume. Total genomic DNA was also used to amplify trnL-trnF fragment and from Jin-Yin-Hua and Shan-Yin-Hua, respectively. The PCR results showed that all of the Jin-Yin-Hua samples contained allele C and all of the Shan-Yin-Hua samples contained allele A (Supporting Information Figure S2).
Loop-mediated isothermal amplification (LAMP)
Fresh Lonicera genus. plants, dried buds (medicinal materials) and twelve patented Chinese drugs were collected (Supporting Information Table S1, S2). The LAMP reaction mixture included 1× ThermoPol® reaction buffer, 0.32 U μl−1 large-fragment Bst DNA polymerase, 0.2 mM dNTPs, 1.6 μM FIP and BIP primers, 0.2 μM F3 and B3 primers and ddH2O. Twenty-four microliters of reaction mixture was pipetted into each well of a 0.2-ml, 96-well PCR plate on ice. Then, 1 μl of each genomic DNA sample was added individually to the appropriate wells. The LAMP reactions were performed by incubating the samples at 63°C for 1 h, heating at 80°C for 10 min to inactivate the reactions and then hold at 4°C. Three negative control reactions (using ddH2O instead of genomic DNA) were performed simultaneously to ensure that the LAMP products were not the result of contamination of reagents with the DNA (Supporting Information Figure S3).
Primer design for genomic LAMP
The online software Primer Explorer version 4.0 (http://primerexplorer.jp/elamp4.0.0/index.html) was used to design LAMP primers. Parameter conditions were as follows: (1) the length of F1c/B1c was 18–23 bp and the length of F2/B2 and F3/B3 was 18–22 bp; (2) the melting temperature (Tm) for F1c/B1c was 60–65°C and Tm for F2/B2 and F3/B3 was 58–61°C; (3) The distance between F2 and B2 was 50–150 bp and the distance between F1c and B1c was 0–50 bp; (4) the single-nucleotide polymorphism (SNP) site was established in the middle of the LAMP primers F2/B2.
Primer design for single-base extension
The single-base extension primer (25–32 bp) was designed using Primer Premier Version 5.0 software (http://www.premierbiosoft.com/crm/jsp/com/pbi/crm/clientside/ProductList.jsp). The SNP site was located at the 3′-terminus of the allele-specific primer. The Tm of the primer was 60–65°C and self-complementarity was avoided.
Digestion of LAMP product
Digestion mixtures (12 μl) were prepared and included 1.2 μl 10× SAP buffer, 1 U exonuclease I, 1 U SAP, 0.05 U pyrophosphatase and 8.3 μl LAMP product. The mixtures was prepared in a 0.2-ml, 96-well PCR plate on ice and were then transferred to a thermocycler and incubated at 37°C for 60 min, heated at 80°C for 10 min and hold at 4°C.
Single-base primer extension
Single-base primer extension mixtures (15 μl) were prepared and included 1.5 μl 10× rTaq buffer, 8.4 pM fluorescein-12-ddUTP, 8.4 pM Cy3-dGTP, 10 μM allele-specific primer, 1 U Taq DNA polymerase and 8.2 μl high-purity water. The mixtures were pipetted into adjacent columns of a 0.2-ml, 96-well PCR plate on ice. Two microliters of digested samples were added individually to the appropriate wells of the PCR plate. The reaction was performed in a 9700 GeneAmp® Thermocycler as follows: initial denaturation at 94°C for 2 min; 3 cycles denaturation at 94°C for 30 s, annealing and extension at 63°C for 30 s and holding at 4°C. The SAP solution included 5 × SAP buffer. SAP (0.5 U μl−1) was prepared and added to each well of the 96-well PCR plate containing the single-base primer-extension products. The plate was transferred to a thermocycler and incubated at 37°C for 20 min, heated at 80°C for 10 min and held at 4°C.
Fluorescence detection
For fluorescence detection by fluorescence resonance energy transfer (FRET) ratio, 558 μl 25 mM HEPES buffer, 36 μl 15 μM PFP and 6 μl single-base primer extension productions was added to a 1.5-ml plastic spectrofluorimeter cuvette. The mixture was vortexed vigorously for 5 s and the emission spectra or intensity of the solution was measured in the cuvette. The FRET ratios of PFP to fluorescein (I530 nm/I425 nm) and of PFP to Cy3 (I574 nm/I425 nm) of the allele-specific extension reactions were plotted pairwise in scatter plots. For visible detection of FRET, 24 μl of PFP was added to the 96-well PCR plate containing single-base primer-extension products and mixed thoroughly by pipetting. The 96-well PCR plate was placed under a UV lamp (ZF-7A) and digital camera (Canon EOS 550D) was used to record the images; the contrast of the images was adjusted so that the inherent fluorescence of the PCR plate was not visible.
Controls
For LAMP, we recommend including three negative control reactions in which high-purity water is used instead of genomic DNA; this ensures that the LAMP products are not the result of contamination of reagents with the DNA. For SNP genotyping using FRET ratios (I530 nm/I425 nm and I574 nm/I425 nm), “blank” single-base extension reactions should be carried out for each allele-specific primer in the absence of target DNA template. The FRET ratio of the blank is used to calculate threshold values.
Detection of botanical origin by fluorescence resonance energy transfer (FRET)
LAMP products were digested by exonuclease I, shrimp alkaline phosphatase (SAP) and pyrophosphatase at 37°C for 60 min, then heated at 80°C for 10 min and hold at 4°C. Single-base primer extension was then conducted in a reaction mixture that included Cy3-dGTP and FI-ddUTP. The SAP solution (2 μl) was added into each well of a 96-well PCR plate containing the single-base primer extension products; the PCR plate was then incubated at 37°C for 20 min, 80°C for 10 min and then hold at 4°C. The single-base primer-extension product (8 μl) was diluted to 600 μl with HEPES buffer in a 1.5-ml fluorimeter cuvette after which the emission spectrum was measured with excitation wavelength 380 nm. The spectrum was measured again after adding 36 μl PFP (15 μM). Changes in fluorescence emission intensity at 425, 530 and 574 nm were recorded to calculate the FRET ratios.
Mixture of Jin-Yin-Hua and Shan-Yin-Hua for screening of botanical origin
Equal amounts of DNA from Jin-Yin-Hua and Shan-Yin-Hua were mixed and 48 samples were prepared for emission measurements. To investigate the dynamic range of the target (Jin-Yin-Hua and Shan-Yin-Hua) DNA, the emission spectra of a series of extension products containing various proportions of each source were measured at excitation wavelength 380 nm after adding PFP.
Screening of patented Chinese drugs
Twelve patented Chinese drugs containing Lonicera buds were used in the study (Qin Re An Chuang Wan, Lin Yan Jie Du Ke Li, Jian Nao Bu Shen, Jin Sang San Jie Wan, Niu Huang Qing Gong Wan, Xiao Er Yan Bian Ke Li, Gan Mao Zhi Ke Ke Li, Lian Qiao Bai Du Wan, Zhi Zi Jin Hua Wan, Qing Guo Wan, Fu Fang Zhen Zhu An Chuang Pian and Xiao Er Gan Mao Ning Tang Jiang). Total DNA was extracted from each plant according to Cui et al27,28. Upon adding PFP to the single-base primer-extension products of these DNA samples, FRET was measured for detection of botanical origin at excitation wavelength 380 nm.
References
Chinese Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China, Part I. 205–206 (China Medical Science and Technology Press, Beijing, 2010).
Chan, T. W. et al. Differentiation and authentication of Panaxginseng, Panax quinquefolius and ginseng products by using HPLC/MS. Anal Chem. 72, 1281–1287 (2000).
Yang, B. et al. GC-MS fingerprints for discrimination of Ligusticum chuanxiong from Angelica. J. Sep. Sci. 31, 3231–3237 (2008).
Joshi, V. C., Avula, B. & Khan, I. A. Authentication of Stephania tetrandra S. Moore (Fang Ji) and differentiation of its common adulterants using microscopy and HPLC analysis. J. Nat. Med. 62, 117–121 (2008).
Shang, X. et al. Lonicera japonica Thunb.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 138, 1–21 (2011).
Wang, H. et al. Molecular authentication of Panax ginseng and ginseng products using robust SNP markers in ribosomal external transcribed spacer region. J. Pharm. Biomed. Anal. 55, 972 (2011).
Lee, O. R., Kim, M. K. & Yang, D. C. Authentication of medicinal plants by SNP-based multiplex PCR. Methods Mol. Bio. 862, 135–147 (2012).
Wang, H. et al. Molecular authentication of the oriental medicines Pericarpium Citri Reticulatae and Citri Unshius Pericarpium using SNP markers. Gene 494, 92–95 (2012).
Xu, G. et al. Authentication of official Da-huang by sequencing and multiplex allele-specific PCR of a short maturase K gene. Genome. 56, 109–113 (2013).
Ding, G. et al. SNP, ARMS and SSH authentication of medicinal Dendrobium officinale KIMURA et MIGO and application for identification of Fengdou drugs. Biol. Pharm. Bull. 31, 553–557 (2008).
Sucher, N. J. & Carles, M. C. Genome-based approaches to the authentication of medicinal plants. Planta Med. 74, 603–623 (2008).
Heubl, G. New aspects of DNA-based authentication of Chinese medicinal plants by molecular biological techniques. Planta Med. 76, 1963–1974 (2010).
Kress, W. J. et al. Use of DNA barcodes to identify flowering plants. Proc. Natl. Acad. Sci. U. S. A. 102, 8369–8374 (2005).
Liu, Z. et al. Applying DNA barcodes for identification of plant species in the family Araliaceae. Gene 499, 76–80 (2012).
Sarwat, M. et al. Molecular markers in medicinal plant biotechnology: past and present. Crit. Rev. Biotechnol. 32, 74–92 (2012).
Jiang, C. et al. Authentication of Lonicera japonica using bidirectional PCR amplification of specific alleles. J. Chin. Mater. Med. 37, 3752–3757 (2012).
Feng, X. et al. Fluorescent logic signal-based multiplex detection of nucleases with the assembly of cationic conjugated polymer and branched DNA. Angew. Chem. Int. Ed. Engl. 48, 5316–5321 (2009).
Feng, F., Liu, L. & Wang, S. Fluorescent conjugated polymer-based FRET technique for detection of DNA methylation of cancer cells. Nat. Protoc. 5, 1255–1264 (2010).
Duan, X., Wang, S. & Li, Z. Conjugated polyelectrolyte–DNA complexes for multi-color and one-tube SNP genotyping assays. Chem. Commun. 1302–1304 (2008).
Duan, X. et al. Single-nucleotide polymorphism (SNP) genotyping using cationic conjugated polymers in homogeneous solution. Nat. Protoc. 4, 984–991(2009).
Duan, X. et al. Cationic conjugated polymers for optical Detection of DNA Methylation, Lesions and Single Nucleotide Polymorphisms. Acc. Chem. Res. 43, 260–270 (2010).
Song, J. et al. Visual detection of DNA mutation using multicolor fluorescent coding. ACS Appl. Mater. Interfaces 4, 2885–2890 (2012).
Notomi, T. et al. Loop-mediated isothermal amplification of DNA. Nucleic. Acids. Res. 28, E63 (2000).
Tomita, N. et al. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat. Protoc. 3, 877–882 (2008).
Han, E. T. Loop-mediated isothermal amplification test for the molecular diagnosis of malaria. Expert. Rev. Mol. Diagn. 13, 205–218 (2013).
Zhang, J. et al. Rapid and sensitive detection of H7N9 avian influenza virus by use of reverse transcription–loop-mediated isothermalamplification. J. Clin. Microbiol. 51, 3760–3764 (2013).
Allen, G. C. et al. A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammonium bromide. Nat. Protoc. 1, 2320–2325 (2006).
Cui, Z. et al. Molecular identification of raw materials from Lian Qiao Bai Du Wan. Acta Pharm Sin. 48, 590–596 (2013).
Acknowledgements
The authors are grateful to the National Natural Science Foundation of China (Nos. 81373959, 81001605 and 21003140) and the Scientific Research Projects of Traditional Chinese Medicine Industry (No. 201407003).
Author information
Authors and Affiliations
Contributions
Y.Y. designed experiments and wrote the paper. C.J. conducted experiments and data analysis. S.Y. and Z.C. collected the majority of the samples provided the case information in this study. M.C. conducted experiments; S.W. designed experiments and revised the paper. L.L. synthesed the polymer CCP and conducted data analysis. S.L. conducted data analysis. L.H. designed experiments, performed data analysis and wrote the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supporting Information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Yuan, Y., Jiang, C., Liu, L. et al. Convenient, Sensitive and High-Throughput Method for Screening Botanic Origin. Sci Rep 4, 5395 (2014). https://doi.org/10.1038/srep05395
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep05395
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.