Abstract
The use of combination drugs is considered to be a promising strategy to control complex diseases such as ischemic stroke. The detection of metabolites has been used as a versatile tool to reveal the potential mechanism of diverse diseases. In this study, the levels of 12 endogenous AAs were simultaneously determined quantitatively in the MCAO rat brain using RRLC-QQQ method. Seven AAs were chosen as the potential biomarkers and using PLS-DA analysis, the effects of the new combination drug YQJD, which is composed of ginsenosides, berberine and jasminoidin, on those 7 AAs were evaluated. Four AAs, glutamic acid, homocysteine, methionine and tryptophan, which changed significantly in the YQJD-treated groups compared to the vehicle groups (P < 0.05), were identified and designated as the AAs to use to further explore the synergism of YQJD. The result of a PCA showed that the combination of these three drugs exhibits the strongest synergistic effect compared to other combination groups and that ginsenosides might play a pivotal role, especially when combined with jasminoidin. We successfully explored the synergetic mechanism of multi-component and provided a new method for evaluating the integrated effects of combination drugs in the treatment of complex diseases.
Similar content being viewed by others
Introduction
Stroke is one of the most common neurological disorders and the third leading cause of death worldwide1. Ischemia causes 87% of stroke and it therefore has become the focus of stroke research2. However, there are no routine, effective, generally accepted and specific treatments for ischemic stroke, except for thrombolysis and endovascular therapies, but their scope is limited especially in developing countries. It is a huge challenge for specific-target drugs or monotherapy to impact the many aspects of clinical trials. The application of combinational drugs, or polypharmacy, in which two or more drugs interact with multiple targets simultaneously, is considered to be a rational and efficient strategy for therapy designed to control stroke. The analysis of metabolites such as endogenous AAs, which can provide global changes of end products in the body, has been used as a versatile tool for the discovery of molecular biomarkers helpful for diagnosing or prognosing clinical disease, exploring the potential mechanism of diverse diseases and assessing the therapeutic effects of drugs3. The subject of metabolomics is whole, dynamic and comprehensive and similar to the nature of Traditional Chinese Medicine (TCM) on the treatment of disease.
TCM, from the viewpoint of holism, always advocates for combined-drug administrations. Over thousands of years, prescriptions of TCM, called formulae, have been made by doctors according to their experience and heritage from their ancestors4. Because of the characteristics of multi-component treatments, research on the use of formulae paid great attention to the integrated pharmacological effects; however, the mechanisms of the synergism are still a mystery due to lack of evidence. The extraction and recombination of the major components of the formulae may help to elucidate the mechanisms of synergism.
The new combination drug Yiqijiedu formulae (YQJD) derived from a homonymous Chinese medicine, which consists of Panax ginseng, Rhizoma Coptidis and Gardenia jasminoides and is widely used to treat brain disease, was studied in this paper. This refined YQJD is composed of ginsenosides (A), berberine (B) and jasminoidin (C), which are the main components of the three herbs in the original formulae. According to the Stroke Therapy Academic Industry Roundtable (STAIR), the infarct volumes, functional response and cerebral blood flow of middle cerebral artery occlusion (MCAO) rat treated with YQJD at 12 and 24 h after MCAO were assessed in our previous study5.
Altered amino acid levels may serve as diagnostic biomarkers for stroke. In the nearly two decades since Benveniste et al.6 demonstrated ischemia-evoked releases of Glu and Asp in the rat hippocampus, the neurochemical processes that occur during cerebral ischemia have been the subjects of numerous studies7,8,9. However, there are many types of neurotransmitters in the brain and the level of neuronal excitability was determined by examining the relative balance between these neuroactive substances. Twelve amino acids that have a direct or indirect relationship with cerebral ischemia in the literature were chosen. Among these, the excessive release of excitatory amino acids such as Glu or Asp was the pathological mechanism behind ischemic brain damage7. Inhibitory AAs, such as γ-aminobutyric acid (GABA) and glycine (Gly), are also released during cerebral ischemia. It has been reported that inhibitory AAs can decrease the severity of ischemic injury and can counteract the toxicity of the excitatory AAs10. Hcy is closely related to stroke and seems to be an independent risk factor for stroke11. Hcy is a sulfhydryl AA product of Met metabolism12. D-serine (D-Ser), an endogenous ligand at the glycine site of N-methyl-D-aspartate (NMDA) receptors, is a major gliotransmitter in the central nervous system13,14. The aromatic AA L-phenylalanine (L-Phe) significantly and reversibly inhibits excitatory glutamatergic synaptic transmission (GST) via a unique set of presynaptic and postsynaptic mechanisms. Trp is metabolized via several pathways and is a precursor for the biosynthesis of the neurotransmitter, serotonin (5-HT), which is a biochemical messenger and regulator15. The role of N-acetyl aspartic acid (Naa) as an indicator of neuronal death originates from the early observation that its level is markedly lower in the area of the cerebral infarction area in stroke16. Other AAs, such as alanine (Ala)17, have been reported to be present at varying levels before and after cerebral ischemia, but their relationships with stroke and the mechanisms involved are not clear.
In the present study, neurological behavior examinations and changes in AA levels were used to compare the efficacy of different combinations of the 3 components to treat cerebral ischemia. It is undoubtedly a new trial to explore the synergetic mechanisms of ginsenosides, berberine and jasminoidin in treating cerebral ischemia by examining the endogenous metabolic changes. Whether this approach could be useful to clarify the integrated therapeutic effects of combination drugs, especially on the treatment of complex diseases, was also investigated.
Results
Determination of cerebral infarct size by magnetic resonance imaging (MRI)
The ischemia produced a marked infarct as a result of the MCAO in the serial MRI coronal brain sections. At 12 h after MCAO, the mean infarct volumes in the vehicle-treated group were 340.94 ± 79.20 mm3 (p < 0.05) (Figure 1). As expected, the oral administration of YQJD (25 mg·kg−1) significantly reduced the infarct volume (p < 0.01) compared to the vehicle control.
Effects of YQJD on the infarct volume of MCAO rats monitored by T2 weighted MRI.
(A) Illustrative MRI images of coronal sections showing the infarct volumes of the cerebral hemisphere as a distinct, bright pale area in the rats subjected to ischemia and the attenuation of the infarct volume by treatment with YQJD; (B) The effects of YQJD on the infarct volume of rats induced by MCAO. YQJD was administered i.g. 15 min prior to MCAO. The values are expressed as the mean ± SD (n = 8) and the data were analyzed with one-way ANOVA. #p < 0.01 versus the sham group; *p < 0.01 versus the vehicle control.
Method validation
To quantitatively and simultaneously determine the concentrations of the 12 endogenous AAs in brain tissue, an triple quadrupole electrospray tandem mass spectrometry (RRLC-QQQ) analytical method was developed and validated. Figure 2 showed the full chromatograms of a mixture of AA standard solutions (100 ng·mL−1, a) and a sample (b). The peaks of each AA were identified by comparing the retention times (Table 1) and using the RRLC-MS/MS chromatograms of the 12 markers shown in Figure 3. All standard curves varied linearly, as shown in Table 2. The correlation coefficient (r2) for all analytes was above 0.9966 indicating good linearity. The LODs were in the range of 0.07–5.31. The results of the calibration curves and the LOD and LOQ values were summarized. The data showing the intra- and inter-day precision and accuracy of the method valuated from the QC samples are summarized in Table 3. The precision of the present method strictly conformed to the criteria for the analysis of biological samples, where the RSD determined at each level did not exceed 15%. The recoveries of the AAs during the sample preparation process were stable and most of the recoveries ranged from 69% to 114%. The recovery for a biological sample must be at least 50% to be acceptable18. The results from all stability tests are presented in Table 4 and demonstrate a good stability of AAs over all steps of the experiment at 4°C. The samples were stable for 7 days at −80°C and the RSD of repeatability did not exceed 8.35%. The method therefore proved to be reliable and applicable for routine analysis.
The improvement of neurological defect by YQJD
MCAO was performed on the left side of rat brains and 12 h later, right hind paresis was observed and compared to the contralateral side. As shown in Figure 4, the mean neurological score of the vehicle-treated group (2.25 ± 0.19) was significantly (p < 0.05, p < 0.01) higher than the sham groups, indicating a neurological defect after the MCAO. However, in the YQJD-treated (ABC of 25 mg·kg−1 and 5 mg·kg−1; AC of 25 mg·kg−1) group, significant improvement of neurological defect was observed compared to the vehicle-treated group.
Effect of YQJD on the neurological deficits induced by MCAO.
The neurological examination scores (behavior test). In the neurological examination, a higher score means a worse pathological condition. The score of each experimental group came from the average of ten subjects' behavioral performances. The values are expressed as the mean ± SD (n = 9) and the data were analyzed using one-way ANOVA; ** p < 0.01 versus the sham group, #p < 0.05 versus vehicle group. Drugs A, B and C represent ginsenosides, berberine and jasminoidin, respectively.
Effect of YQJD on the changes in amino acid levels in rat brain tissue
The levels of 12 endogenous AAs (Glu, Trp, Phe, Tyr, Met, GABA, Asp, Ser, Ala, Naa, Gly and Asp) in ischemic brains were analyzed at 12 h after the MCAO. Our results show that the concentrations of 11 amino acids were increased in the tissue after MCAO, but the Naa level dropped significantly.
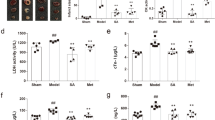
First, a supervised partial least squares discriminate analysis (PLS-DA) model with the 12 AAs successfully discriminated between the sham and vehicle groups, with high fitness (R2 value) and prediction power (Q2 value). The results indicate that the PLS-DA model had an R2 value of 0.959 and a Q2 value of 0.94 at 12 h after MCAO. The amino acids that had remarkably altered concentrations between these two groups as determined by PLS-DA (VIP > 1) were chosen for further study. A PLS-DA score plot (Figure 5) showed a clear differentiation between these two groups at 12 h after MCAO, suggesting that marked changes in the levels of amino acids occurred during the ischemic injury. According to the PLS-DA results, 7 AAs (Naa, Ala, Glu, Asp, GABA, Gly, Ser) were potential biomarkers. At 12 h after MCAO, there were significant differences (p < 0.05, p < 0.01) in these seven AAs between the vehicle and sham groups, as shown in Figure 6. Moreover, the levels of Glu and GABA changed significantly in all of the YQJD-treated groups and the concentration of four AAs (Ala, Asp, Gly and Ser) significantly decreased in the two ABC (25 mg·kg−1 and 5 mg·kg−1) treated groups compared to the vehicle groups (p < 0.05, p < 0.01).
Effect of YQJD on the levels of 7 amino acids (ng·mL−1).
(a) GABA, (b) Ala, (c) Gly, (d) Asp, (e) Ser, (f) Glu, (g) Naa; the values were analyzed using one-way ANOVA and expressed as the mean ± SD (n = 9). **p < 0.01,*p < 0.05 versus the sham group; ##p < 0.01, #p < 0.05 versus the vehicle group. Drugs A, B and C represent ginsenosides, berberine and jasminoidin, respectively.
To investigate the combinational effects of A, B and C, a principal component analysis (PCA) was used to analyze the AA level data in the different groups. The rate of accumulation of the previous five principal components reached 86% at the dosages of 5 and 25 mg·kg−1 (Figure 7). At the dosage of 25 mg·kg−1, the ABC group showed the best performance in PC 1 and PC 2, which was closer to the normal level (Figure 8 a). At the dosage of 5 mg·kg−1, the ABC and only A treated groups had relatively good performance in the first two principal components (PC 1 and PC 2), respectively (Figure 8 b). The ABC treatment seemed to be consistent at both dosages in the PC1 s.
The association between the values of each PC and the various drugs combinations (a: the PCs at the dosage of 25 mg·kg−1; b: the PCs at the dosage of 5 mg·kg−1).
At the dosage of 25 mg·kg−1, the ABC group had the best performance in PC1 and PC2 and was close to the level of the sham group (Figure 8 a). At the dosage of 5 mg·kg−1, the ABC- and only A-treated groups performed relatively well in the first two principal components (PC 1 and PC 2), respectively (Figure 8 b). Drugs A, B and C represent ginsenosides, berberine and jasminoidin, respectively.
Discussion
With the improvement of new drug research and development (R&D), drugs with a high selectivity to a single target have shown their limitations. The pattern of single target drug treatment seemed to be tough to meet the requirement of the treatment for complex diseases. Multi-component therapeutics, in which two or more agents interact with multiple targets simultaneously, is a rational and efficient form of therapy designed to control complex diseases19,20. Notably, combination therapies and systematic theories have been used for more than 2500 years in traditional Chinese medicine (TCM) to prevent and cure diseases and have accumulated a large number of clinical experiences. There is no doubt that TCM is an available and noteworthy source to develop new combination drugs from which, at least in some formulae, multiple components could hit multiple targets and exert synergistic therapeutic efficacies21. Researchers have made good attempts to explain and identify the combinational roles of multi-component therapeutics from Chinese medicines22. However, more studies are still needed to verify these mechanisms. Here, we have shown that a new YQJD combination drug from TCM offers protective effects on cerebral ischemia. To further understand the synergistic effects and combinational rules, we designed seven different combinations of three drugs to treat cerebral ischemia in a rat MCAO model. From the behavioral scores, the combination groups, such as ABC and AC, exhibited remarkable neuroprotective activity.
In the present study, we established a simple method for the simultaneous detection of 12 underivatized AAs based on RRLC-MS/MS technology. Using metabolic AA profiling, a PLS-DA model was developed to discriminate between the sham and MCAO operated groups and seven AAs (Naa, Ala, Glu, Asp, GABA, Gly and Ser) with VIP > 1 in the brain tissue were selected as potential biomarkers. The ANOVA results showed that there were significant differences in the levels of the seven potential biomarkers between the vehicle-treated and sham groups, which is in accordance with the PLS-DA results. However, the levels of four biomarkers (Ala, Asp, Gly and Ser) were also altered in all the drug-treated groups, especially in the 5 and 25 mg·kg−1 ABC treated groups. Although the analysis found that Ala, Asp, Gly and Ser were the key biomarkers in the YQJD treatment of cerebral ischemia, it did not determine the mechanism of combinational effects.
To further investigate the combinational effects of the drugs, we divided the representative features of multiple AAs into several functional groups that were sensitive to different combinations of drugs. In bioinformatics, PCA is an efficient and effective feature selection method that has been widely used in a variety of scientific fields to extract common patterns from multiple observation samples. In the present study, the top two principal components (PCs), the 5 and 25 mg·kg−1-dosage groups, could explain more than 86% of variation in AAs in the different experimental groups and were selected for analysis. In the 25 mg·kg−1-dosage group, PC1 and PC2 had similarities in quantities and distributions, that is, the PCA values of the ABC-treated groups were all consistent with normal levels. At the dosage of 5 mg·kg−1, the drugs ABC and A had good performances in the two principal components (PC1 and PC2). Thus, the ABC experiment groups had the best performance, followed by the AC group. Component A, ginsenosides, a type of steroidal glycoside, has many pharmacological effects on the central nervous, endocrine, immune and cardiovascular systems23,24,25, which could improve the overall state of the organism. Moreover, ginsenoside Rd was reported to protect cultured hippocampal neurons against glutamate-induced excitotoxicity after focal cerebral ischemia and this neuroprotective effect may result from the inhibitory effects of GSRd on Ca2+ influx26. Component C, jasminoidin, a type of glycoside, has been proven to have positive effects on cerebral ischemia through inflammation inhibition and the release of excitatory neurotransmitters27. Berberine, a major natural morphinane alkaloid constituent of the Chinese herb Coptidis Rhizoma, has been shown to exert potent antitumor, anti-inflammatory, antidiarrheal and antidiabetic effects28,29,30. The synergistic effects of ABC may be closely related to the complementary structure of the three components. The results of neuroprotective activity and PCA analysis suggest that component A has a clear positive effect on MCAO rats and it may be the critical component of YQJD. This study also shows that PCA might be an appropriate method to analyze the complicated data obtained in the study of combination drugs.
In conclusion, this study first presented the synergism and combinational rules of YQJD in the treatment of cerebral ischemia from the perspective of AA metabolism. Understanding the synergistic mechanisms of multi-component drugs is critical for developing novel strategies to combat complex diseases. The purpose of this study was to search for a reasonable tool to clarify the role and rules of combination drugs by evaluating the changes in the endogenous AA metabolite profile, together with successfully simulating the integrated therapeutic model by determining the principal components. This study provides a new method to investigate and understand the molecular mechanism of TCM and it could be a promising strategy to explain combination theory of multi-component drugs based on the viewpoint of holism.
Methods
Chemicals and reagents
The AA standards, L-glycine (Gly), alanine (Ala), γ-aminobutyric acid (GABA), L-aspartic acid (Asp), L-glutamic acid (Glu), L-methionine (Met), L-phenylalanine (Phe), tyrosine (Tyr), D-serine (D-Ser), homocysteine (Hcy), N-acetyl aspartic acid (NAA) and DL-tryptophan (Trp) were purchased from Sigma-Aldrich Co., Ltd.(Beijing, China) The formic acid (purity 99%) was obtained from Roe Scientific Inc. (Newark, DE, USA). All solutions were prepared using LC-MS Ultra High Purity water and LC-MS-grade acetonitrile (Tedia Company Inc., USA). The I.S. (acrylamide-d3) was purchased from Toronto Research Chemicals Inc. (Brisbane, North York, Canada).
The ginsenosides (Rg1+Re+Rd ≥ 40.55 ± 2.10%) were purchased from Nanjing ZeLang Medical Technology Co., Ltd. (Nanjing, China). The berberine (Purity ≥ 95.18%) was purchased from Xianyang Aviation 168 Bio-Engineering Co., Ltd (Xianyang, China). The jasminoidin (purity ≥ 99.68%) was purchased from Baoji F.S. Biological Development Co., Ltd (Baoji, China).
Animal handing procedure
All animal experiments were performed on male Sprague-Dawley rats weighing 250–270 g and obtained from Beijing Vital River Company (P.R. China). All animals were housed individually at 22 ± 2°C and a relative humidity of 50 ± 10% with a 12 h light/dark cycle and free access to chow and water. The rats were adapted to the housing conditions for 2 days before the experiments. The animals were taken care of by the China Academy of Chinese Medical Sciences' Laboratory Animal Care Center. All animal experiments were performed in accordance with institutional guidelines and ethics. The Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences approved all animal experiments performed in this study.
The middle cerebral artery occlusion (MCAO) model in rats has been widely used since 197531,32. This technique is widely accepted33 and has become a classical method in the research of focal cerebral ischemia. To ensure the success of the MCAO procedure, the MCAO operation using the intraluminal filament method was performed by one experienced researcher according to a previous method by Zea Longa with some slight modifications34. The rectal temperature was recorded and maintained at 37 ± 0.5°C throughout the surgical procedure. Animals were anesthetized with chloral hydrate (10%, 400 mg·kg−1, i.p.) and placed in a supine position on an operation table. A midline neck incision was made. The left common carotid artery, the left external carotid artery (ECA) and the left inner carotid artery were exposed. A fishing thread (diameter of 0.26 mm) lightly dipped in paraffin was inserted into the left common carotid artery and occluded the middle cerebral artery. Animals in the sham group underwent the same procedures as described above with the exception of the insertion of the nylon filament into the inner carotid.
In this experiment, we designed the experiment to prove whether the three components used together would represent the maximum pharmacological effect. Two doses were used: 5 mg·kg−1 and 25 mg·kg−1. For each dose, various combinations consisting of one, two or three components were used. Sixteen experiments were conducted as explained in Table 5. Each group was given YQJD intragastrically (i.g.) 15 min prior to MCAO. The sham and vehicle-treated rats were given physiological saline intragastrically.
All 196 rats were randomly divided into 16 groups with 12 rats each, the dead and unsuccessful rats were excluded and the remaining rats were used in the final analysis.
Cerebral infarct size by magnetic resonance imaging (MRI)
Magnetic resonance imaging (MRI) is an invaluable tool used in the diagnosis of ischemic stroke and can therefore determine the extent of ischemic injury within the initial hours of ischemia35. Conventional T2-weighted MRI becomes sensitive to ischemic changes only after a net increase in the water content of the cerebral tissue and therefore can detect ischemia a few hours after symptom onset and play a role in estimating the final tissue outcome, specifically by providing information on severity and location of the ischemic insult. This classical MRI detection was used in this study to evaluate the therapeutic effects of YQJD treatment after focal cerebral ischemia in rats. In vivo MRI was performed on a 7T MRI animal scanner (Varian, Palo Alto, CA). The 16-cm horizontal bore magnet equipped with actively shielded gradients capable of 200 mT/m used a receive-only head surface coil (Rapid Biomedical, Rimpar, Germany) that was actively decoupled from a transmit birdcage resonator 12 h after MCAO.
Rat brains were imaged in vivo with a T2-weighted fast spin-echo (T2FSE) sequence. The imaging parameters were repetition time (TR) = 3000 ms; echo time (TE) = 72 ms; slice thickness = 1.5 mm; Gap = 0.5; NEX = 4; and image matrix size = 256 × 256, covering the whole brain with a field-of-view (FOV) of 34.8 mm × 43.3 mm, which resulted in a final in-plane resolution of 135 μm × 169 μm. The quantitative measurement value of the infarct area of each slice was extracted from the MRI data to compare the YQJD-treated to the vehicle-treated animals. Surgery failure, defined by the absence of lesions on the MRI, was an exclusion criterion. The adjusted infarct areas of each slice were determined by image analysis software (Vnmrj 4.0). The infarct volume of each rat was calculated as the infarct area × thickness (1.5 mm). The sum of the infarct volumes of all the brain slices was the total infarct volume.
Assessment of neurological defects
Neurological deficit was evaluated at 12 h after the MCAO procedure. The time point of 12 h after MCAO is pivotal in acute cerebral ischemia36,37 and the levels of some neurotransmitters had changed significantly at that point, especially Glu and Gly38,39. The effects of the YQJD formulae at that point had been verified with other indicators, such as the cerebral infarct volume, cerebral edema and mitochondrial function5. The neurological scores were assessed as described by Huang et al.40 and then the animals were euthanized. The researcher was blinded to the experimental treatment groups. The neurological scores were defined as follows: 0, no neurological deficit; 1, failure to extend the right forelimb; 2, circling to the contralateral side; 3, falling to the contralateral side at rest; and 4, no spontaneous motor activity.
Brain tissue homogenate sampling and preparation
The left brain hemispheres were weighed and homogenized in 5 mL initial mobile phase solution (90% water with 0.1% formic acid and 10% acetonitrile) in the cold (0°C) using a homogenizer. The brain homogenate was collected in a 10-mL centrifuge tube and then centrifuged at 3000 rpm for 10 min at 4°C. The separated homogenate was kept in the dark at −80°C until analysis. Next, 25 μL of the homogenate specimen was pipetted into a 1.5-mL eppendorf tube and spiked with 10 μL of I.S. stock solution (the final concentration is 1000 ng·mL−1) and then initial mobile phase was added to a final volume of 500 μL. After mixing with a vortexer for 1 min, the mixture (500 μL) was filtered before the analysis.
Instrumentation (chromatographic and MS spectrum conditions)
Rapid resolution liquid chromatography (RRLC) analysis was performed on an Agilent 1200 rapid resolution liquid chromatographer (Santa Clara, CA USA) equipped with an online vacuum degasser, a binary pump, an autosampler and a thermostatted column compartment. The analytes were separated on a Diamonsil C18(2) column (5 μm, 250 × 4.6 mm) at a column temperature of 30°C. The mobile phase for elution was a gradient established between solvent A (water containing 0.1% formic acid) and solvent B (acetonitrile) at a flow rate of 0.5 mL·min−1. Baseline separation was achieved using a gradient starting from 90% A/10% B followed by a linear increase in B reaching 50% B at 10 min. Then, the eluent was returned to the initial conditions over 10.20 min, where it remained until the end of the run to allow equilibration.
An Agilent G6410 triple quadrupole mass spectrometer equipped with an electrospray ion (ESI) source (Agilent, MA, USA) was operated in positive ion mode using MRM scanning. The following optimal conditions were used: electrospray capillary voltage, 4000 V; nebulizer pressure, 45.0 psi; drying gas, nitrogen; flow rate, 11 L/min; and temperature, 350°C. High-purity nitrogen was used as the collision gas. To establish the appropriate MRM conditions, standard solutions were infused into the MS for optimization. The acquisition parameters for each analyte are listed in Table 1 and the fragmentor voltage (FV) and collision energy (CE) varied between the different markers. Agilent Mass Hunter workstation software version B.01.04 was used for data acquisition and processing.
Identification of the endogenous AAs
The RRLC/QQQ analytical method was developed and validated for the simultaneous quantification of 12 AAs in rat brain tissue. The method was validated by determining the linearity, inter- and intra-assay precision, recoveries, sensitivity and stability (Supplementary Text S1). The calibration was performed using a least-squares linear regression of the peak area ratios of the AAs to the I.S. versus the respective standard concentrations. The sensitivity was evaluated with the limit of detection (LOD) and the limit of quantification (LOQ) of the transitions of each compound. The LOD and LOQ were obtained at the concentration that provided signal-to-noise ratios of 3 and 10. The intra- and inter-day precision were evaluated using QC samples at three different concentrations. The intra-day precision was assessed by the analysis of the AAs in the QC samples (n = 6) on the same day. The inter-day precision was evaluated by the repeated analysis of the AAs in the QC samples over three consecutive days. The recovery was determined as the ratio of the concentration measured versus the sum of the nominal concentration added into the brain homogenate and the initial concentration of AAs in brain tissue. The stability of the AAs in the brain tissue was assessed by analyzing the replicates (n = 6) of samples during the same storage and processing procedures. Collected tissue samples were analyzed with commercially purchased standards.
Data analysis
All data were acquired and processed using Agilent Mass Hunter workstation software version B.01.04 (Agilent, MA, USA). The quantitative data are presented as the mean ± SD. The data from the vehicle groups was compared with the sham groups and the data from the experimental groups was compared with the vehicle groups. The Kolmogorov-Smirnov test was used to determine the normality of the distribution of continuous variables. A one-way ANOVA was used to compare the 12 variables in nine groups. The least significant difference (LSD) was used for the post hoc test. Additionally, the power analysis was used to test the sample size with STATISTICA software. On one hand, the power of our sample was near to 80% according to Cohen (1983)41. On the other hand, we set 5% as the significance level and 77% as our power goal. The result showed that the required sample size was 12, which was consistent with our sample size. According to the power analysis, our sample size was large enough to test the hypotheses of our study.
To investigate the combinational effects of A, B and C, PCAs were used to analyze the AA profiles with SPSS (Statistical Package for the Social Sciences) 18.0 software. PLS-DA was used to explore the differences between the sham and vehicle groups. A variable importance in projection (VIP) > 1, which was selected as the cutoff value to find the most important variables42, was used to extract novel potential biomarker ions with the PLS-DA model. The PLS-DA analyses were applied using SIMCA-P 12.0 software (Umertrics, Umea, Sweden).
References
Strong, K., Mathers, C. & Bonita, R. Preventing stroke: saving lives around the world. Lancet Neurol. 6, 182–187 (2007).
Lloyd-Jones, D. et al. Heart disease and stroke statistics-2009 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 119, e21–e181 (2009).
Brindle, J. T. et al. Rapid and noninvasive diagnosis of the presence and severity of coronary heart disease using 1 H-NMR based metabonomics. NAT. MED. 8, 1439–1444 (2002).
Wang, L. et al. Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. Proc. Natl. Acad. Sci. USA. 105, 4826–4831 (2008).
Shaojing, L. et al. An Effective Solution to Discover Synergistic Drugs for Anti-Cerebral Ischemia from Traditional Chinese Medicinal Formulae. Plos one. 11, e78902 (2013).
Benveniste, H., Drejer, J., Schousboe, A. & Diemer, N. H. Elevation of the Extracellular concentrations of glutamate and aspartate in rat hippoc-ampus during transient cerebral ischemia monitored by intracerebral microdialysis. J. NEUROCHEM. 43, 1369–1374 (1984).
Saransaari, P. & Oja, S. S. Mechanisms of ischemia-induced taurine release in mouse hippocampal slices. BRAIN RES. 807, 118–124 (1998).
Phillis, J. W. & O'Regan, M. H. Characterization of modes of release of amino acids in the ischemic/reperfused rat cerebral cortex. NEUROCHEM. INT. 43, 461–467 (2003).
Adachi, N. Cerebral ischemia and brain histamine. BRAIN RES REV. 50, 275–286 (2005).
Wan, H. & Zhao, T. Effects of gastrodin on amino acids after cerebral ischemia-reperfusion injury in rat striatum. Asia Pac. J. Clin. Nutr. 16, 305–308 (2007).
Faraci, F. M. & Lentz, S. R. Hyperhomocysteinemia, oxidative stress and cerebral vascular dysfunction. Stroke. 35, 345–347 (2004).
Pettigrew, L. C., Bang, H., Chambless, L. E., Howard, V. J. & Toole, J. F. Assessment of pre- and post-methionine load homocysteine for prediction of recurrent stroke and myocardial infarction in the Vitamin Intervention for stroke Prevention trial. Atherosclerosis. 200, 345–349 (2008).
Yang, Y. et al. Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proc. Natl. Acad. Sci. USA. 100, 15194–15199 (2003).
Stevens, E. R. et al. D-serine and serine racemase are present in the vertebrate retina and contribute to the physiological activation of NMDA receptors. Proc. Natl. Acad. Sci. USA. 100, 6789–6794 (2003).
Gao, X. et al. Metabonomic study on chronic unpredictable mild stress and intervention effects of Xiaoyaosan in rats using gas chromatography coupled with mass spectrometry. J. Ethnopharmacol. 137, 690–699 (2011).
Frahm, J. et al. Localized high-resolution proton NMR spectroscopy using stimulated echoes: initial applications to human brain in vivo. Sauter. Magn. Reson. Med. 9, 126–131 (1989).
Zhang, Z. X., Gao, P. F., Guo, X. F., Wang, H. & Zhang, H. S. 1,3,5,7-Tetramethyl-8- (N- hydroxysuccinimidyl butyric ester) difluoroboradiaza-s-indacene as a new fluorescent labeling reagent for HPLC determination of amino acid neurotransmitters in the cerebral cortex of mice. Anal. Bioanal. Chem. 401, 1905–1914 (2011).
Zhu, K. Y. et al. The establishment of a sensitive method in detecting different neurotransmitters simultaneously in brains by using liquid chromatography electrospray tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 879, 737–742 (2011).
Carey, K. M., Comee, M. R., Donovan, J. L. & Kanaan, A. O. A polypill for all Critical review of the polypill literature for primary prevention of cardiovascular disease and stroke. Ann. Pharmacother. 46, 688–695 (2012).
Zimmermann, G. R., Lehar, J. & Keith, C. T. Multi-target therapeutics: when the whole is greater than the sum of the parts. Drug Discov. Today. 12, 34–42 (2007).
Wang, L. et al. Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. Proc. Natl. Acad. Sci. USA. 105, 4826–4831(2008).
Li, S., Zhang, B. & Zhang, N. Network target for screening synergistic drug combinations with application to traditional Chinese medicine. BMC Syst. Biol. 5 Suppl 1, S10 (2011).
Gillis, C. N. Panax ginseng pharmacology: a nitric oxide link? BIOCHEM. PHARMACOL. 54, 1–8 (1997).
Attele, A. S., Wu, J. A. & Yuan, C. S. Ginseng pharmacology: multiple constituents and multiple actions. BIOCHEM. PHARMACOL. 58, 1685–1693 (1999).
Lü, J. M., Yao, Q. & Chen, C. Ginseng compounds: an update on their molecular mechanisms and medical applications. CURR. VASC. PHARMACOL. 7, 293–302 (2009).
Zhang, C. et al. Ginsenoside Rd protects neurons against glutamate-induced excitotoxicity by inhibiting Ca2+ influx. Cell Mol. Neurobiol. 32, 121–8 (2012).
Li, J. et al. Synergism and Rules from Combination of Baicalin, Jasminoidin and Desoxycholic acid in Refined Qing Kai Ling for Treat Ischemic Stroke Mice Model. PLOS ONE. 7, e45811 (2012).
Ku, S. K. et al. Effect of Curculigo orchioides on reflux esophagitis by suppressing proinflammatory cytokines. Am. J. Chin. Med. 40, 1241–1255 (2012).
Compare, D. et al. Effects of long-term PPI treatment on producing bowel symptoms and SIBO. Eur. J. Clin. Invest. 41, 380–386 (2011).
Allescher, H. D. & Wagner, H. STW 5/Iberogast: multi-target-action for treatment of functional dyspepsia and irritable bowel syndrome. Wien Med Wochenschr. 157, 301–307 (2007).
Robinson, R. G., Shoemaker, W. J., Schlumpf, M., Valk, T. & Bloom, F. E. Effect of experimental cerebral infarction in rat brain on catecholamines and behavior. Nature 255, 332 (1975).
Robinson, R. G. Differential behavioral and biochemical effects of right and left hemispheric cerebral infarction in the rat. Science 205, 707–710 (1979).
Tamura, A., Graham, D. I., McCulloch, J. & Teasdale, G. M. Focal Cerebral Ischaemia in the Rat: 2. Regional Cerebral Blood Flow Determined by [14C] Iodoantipyrine Autoradiography Following Middle Cerebral Artery Occlusion. J. Cereb. Blood. Flow. Metabol. 1, 61–69 (1981).
Wang, L. et al. Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. Proc. Natl. Acad. Sci. USA. 105, 4826–4831 (2008).
Arsava, E. M. The Role of MRI as a prognostic tool in ischemic stroke. J Neurochem. 123 Suppl 2, 22–28 (2012).
Mohammadi, M. T., Shid-Moosavi, S. M. & Dehghani, G. A. Contribution of nitric oxide synthase (NOS) in blood-brain barrier disruption during acute focal cerebral ischemia in normal rat. Mohammad T. Pathophysiology. 19, 13–20 (2012).
Wu, L. et al. Keep warm and get success: The role of postischemic temperature in the mouse middle cerebral artery occlusion model. Brain Res Bull. 101, 12–17 (2013).
Dávalos, A., Shuaib, A. & Wahlgren, N. G. Neurotransmitters and Pathophysiology of Stroke: Evidence for the Release of Glutamate and Other Transmitters/Mediators in Animals and Humans. Journal of Stroke and Cerebrovascular Diseases 9, 2–8 (2000).
Gao, J. et al. Analysis of serum metabolites for the discovery of amino acid biomarkers and the effect of galangin on cerebral ischemia. Mol Biosyst. 9, 2311–2321 (2013).
Huang, Z. et al. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science 265, 1883–1885 (1994).
Jacob, C. Statistical power analysis for the behavioral sciences [273–406] (Hillsdale, New Jersey, 1988).
Wold, S., Sjostrom, M. & Eriksson, L. PLS-regression: a basic tool of chemometrics. Chemom. Intell. Lab. Syst. 58, 109–130 (2001).
Acknowledgements
This research was supported by grants from the National Natural Science Foundation of China (No. 81274133 and 81303261) and the Major Scientific and Technological Special Project for “Significant New Drugs Creation” (No. 2012ZX09103-201-055).
Author information
Authors and Affiliations
Contributions
S.J.L., J.G. and C.C. planned and performed the experiments, collected and analyzed the data and wrote the paper. J.X.C. analyzed the results. All the other authors including L.M.W., G.L.Y., F.P.D., H.Z.Y., L.D.F., D.R.X. and H.J.Y. discussed the results and commented on the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary materials
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. The images in this article are included in the article's Creative Commons license, unless indicated otherwise in the image credit; if the image is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the image. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Gao, J., Chen, C., Chen, JX. et al. Synergism and Rules of the new Combination drug Yiqijiedu Formulae (YQJD) on Ischemic Stroke based on amino acids (AAs) metabolism. Sci Rep 4, 5149 (2014). https://doi.org/10.1038/srep05149
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep05149
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.