Abstract
Male BALB/c mice fed with either a regular or high fat diet were exposed to 0, 5 or 20 mg/kg perfluorooctane sulfonate (PFOS) for 14 days. Increased body weight, serum glucose, cholesterol and lipoprotein levels were observed in mice given a high fat diet. However, all PFOS-treated mice got reduced levels of serum lipid and lipoprotein. Decreasing liver glycogen content was also observed, accompanied by reduced serum glucose levels. Histological and ultrastructural examination detected more lipid droplets accumulated in hepatocytes after PFOS exposure. Moreover, transcripitonal activity of lipid metabolism related genes suggests that PFOS toxicity is probably unrelevant to PPARα's transcription. The present study demonstrates a lipid disturbance caused by PFOS and thus point to its role in inhibiting the secretion and normal function of low density lipoproteins.
Similar content being viewed by others
Introduction
Perfluorooctane sulfonate (PFOS) is a ubiquitous perfluorinated compound with a variety of uses in both industrial and consumer products, leading to its increased global exposure1,2,3. It can now be detected in the liver and blood of fish, birds and mammals, even in human serum and milk2,4,5,6. Though the PFOS concentration in general population and wildlife was at the ng/mL level, concentrations over 10 μg/mL have been detected in serum of occupational populations7,8. And in vivo studies revealed that exposure to 10 and 20 mg/kg PFOS could affect the neuroendocrine system or cause lung injury in rats9,10; 5, 20 and 40 mg/kg PFOS treatment induced reproductive or immune abnormalities in mice11,12,13.
The liver is an important organ for detoxification and lipid metabolism. As the primary site of bioaccumulation of certain pollutants, it is likely to be a target of PFOS14. Previous studies reported that PFOS decreased body weight of rodents while conversely increased the liver index9,13,15, intracellular hepatic fatty acid and cholesterol content16. Vacuolation and hypertrophy of hepatic cells also occurred in PFOS-treated mice and rats15,17,18. PFOS has been considered to disturb the expression of hepatic genes associated with fatty acid synthesis, activation, transport and oxidation pathways, as well as hormonal regulation14,19,20. Accordingly, PFOS-treated rodents got reduced serum cholesterol and triglyceride levels16,18,21, decreased thyroid hormone concentrations and elevated levels of serum corticosterone8,22,23.
Peroxisome proliferator-activated receptor α (PPARα) is responsible for regulating the expression of genes involved in fatty acid, cholesterol metabolism and DNA replication as well24,25. PFOS induces PPARα activation in both rodents and humans, this has been supposed to be a key factor in its various toxicities26,27,28. As PFOS resembles fatty acid in structure, it can bind to apolipoprotein and disturb lipid transport thus affect the physiological effects of lipids, this might also contribute to PFOS-caused toxicities29. However, those hypotheses are still under debate, the specific mechanism needs to be elucidated.
Feeding a high fat diet (HFD) to rodents causes increase in body weight, fat mass accumulation and circulating concentrations of lipids and accelerates free fatty acid metabolism30,31,32. In this study, male BALB/c mice were fed either a regular diet (RD) or a HFD during PFOS exposure. Considering that PFOS is structurally similar with fatty acid, a HFD here could mobilize more lipids and might relieve the competition of PFOS. Furthermore, by comparing the results of HFD-fed and RD-fed mice, we aimed to explore the molecular mechanism of PFOS-induced toxicity.
Results
Body weight and organ indices
Though consuming comparable feed, HFD-fed controls gained more weight than RD-fed ones (4.12 g compared to 2.46 g) after 14 days' exposure; the extra fat also caused increasing weight of the livers and ventral fat (Table 1). 5 mg/kg PFOS had no effect on body weight change or feed consumption of RD-fed mice, but caused body weight loss in individuals fed a HFD (P < 0.05). Daily feed consumption of 20 mg/kg PFOS-treated mice was reduced, which was more significant in RD-fed animals. Decreased body weight was also observed in those individuals (P < 0.01, Table 1).
In contrast to the body weight loss, PFOS-exposed livers were more hypertrophic, in a dose-dependent manner (P < 0.01, Table 1). Therefore the liver indices were significantly elevated (P < 0.01, Table 1). However, there was a decrease in the amount of ventral fat in PFOS-exposed mice. This was significant when treated with 20 mg/kg PFOS (P < 0.01), no or little ventral fat was obtained (Table 1). Accordingly, the fat indices of these mice were decreased with the increasing PFOS doses. Both RD and HFD-fed mice displayed similar changes (Table 1).
Hepatic fat and glycogen content
As enlarged livers occurred after PFOS exposure, liver fat content was determined later to assess hepatic lipid accumulation (Fig. 1). For control mice, more fat existed in the livers when fed a HFD (P < 0.01). Consistent with the increased liver indices, a significant increase in liver fat content occurred in PFOS-treated RD individuals (P < 0.01). No significant increase was observed in HFD-fed mice after PFOS exposure, but they still retained higher levels than RD-fed controls (Fig. 1).
Liver glycogen, acting as a storage form of glucose and maintaining steady blood glucose levels, was also detected. Though ingesting more fat, no obvious change of liver glycogen was observed in HFD-fed control mice. Reduced liver glycogen content occurred in PFOS-exposed RD mice (Fig. 1). HFD-fed mice got similar decrease following PFOS exposure, more obvious in 5 mg/kg dose group (2.45 mg/g liver compared to 6.17 mg/g liver, P < 0.01).
Histological and ultrastructural assessment of the liver
H & E stained liver sections showed hydropic degeneration and vacuolation in the hepatocytes after PFOS exposure and more severe in 20 mg/kg dose groups (Fig. 2-B, C, E and F). HFD-fed mice demonstrated greater susceptibility to pathological changes of hepatocytes than RD individuals, with significantly more hypertrophied hepatic cells and enlarged intercellular spaces (Fig. 2-D, E and F). However, the nuclei remained unaffected after PFOS treatment (Fig. 2-B, C, E and F).
H & E stained sections of livers from control and PFOS-exposed mice.
Sections of livers viewed under light microscopy. (A) Control mice fed a regular diet, (B) 5 mg/kg PFOS-treated mice fed a regular diet, (C) 20 mg/kg PFOS-treated mice fed a regular diet, (D) control mice fed a high fat diet and (E) 5 mg/kg PFOS-treated mice fed a high fat diet, (F) 20 mg/kg PFOS-treated mice fed a high fat diet. Scale bar in panel A, 200 μM.
The ultrastructure of hepatocytes was observed by using a transmission electron microscopy. More lipid droplets were accumulated in hepatic cells of HFD-fed control mice (Fig. 3-A, D). Some PFOS-exposed RD mice had voids within the cytoplasm resulting from dilatations of the endoplasmic reticulum (Fig. 3-B, C), these were also observed in HFD-fed ones (Fig. 3-E, F). More lipid droplets accumulated in livers of RD and HFD-fed mice following PFOS exposure (Fig. 3-B, C, E and F), especially in 20 mg/kg dose groups (Fig. 3-C, F). This is consistent with their histological changes and increasing fat content of the livers.
Hepatocellular ultrastructure in control and PFOS-exposed mice.
Transmission electron microscopy images of representative liver samples. (A) Control mice fed a regular diet, (B) 5 mg/kg PFOS-treated mice fed a regular diet, (C) 20 mg/kg PFOS-treated mice fed a regular diet, (D) control mice fed a high fat diet and (E) 5 mg/kg PFOS-treated mice fed a high fat diet, (F) 20 mg/kg PFOS-treated mice fed a high fat diet. L = Lipid droplet. Scale bar is shown in each panel.
Moreover, sections of kidney, heart and intestine showed no obvious pathological changesafter PFOS treatment, which verified that these organs were not the main target of PFOS.
Changes in serum biochemical parameters
Comparing with RD mice, a HFD induced higher serum glucose level in control groups (Fig. 4-A), but the liver glycogen content retained a comparable level. For both RD and HFD-fed mice, serum glucose level remained unchanged when treated with 5 mg/kg PFOS, but was decreased significantly in 20 mg/kg dose groups (P < 0.01, Fig. 4-A). Serum albumin concentration, a reflection of liver function, was not changed by a HFD; While it increased significantly in a dose-dependent manner following PFOS exposure (P < 0.01 for both RD and HFD-fed mice, Fig. 4-B).
Serum biochemical parameters after 14 days of PFOS exposure.
The legend and numbers of mice per group are detailed in panel A. *P < 0.05, **P < 0.01 versus their respective controls or between the two groups indicated, using one-way ANOVA. RD = regular diet, HFD = high fat diet. All values are means ± SE (standard error).
Despite the high fat in diet, serum triglyceride in HFD control mice retained similar levels with RD controls (Fig. 4-C). However, the HFD caused elevated levels of serum cholesterol (P < 0.01, Fig. 4-D). High-density lipoprotein cholesterol (HDL-ch) and low-density lipoprotein cholesterol (LDL-ch), according with HDL and LDL, their levels were also increased by feeding a HFD (P < 0.01, Fig. 4-E, F).
For RD-fed mice, serum triglyceride level was slightly elevated after 5 mg/kg PFOS exposure, while decreased significantly in 20 mg/kg dose group (P < 0.01, Fig. 4-C). HFD-fed mice displayed similar changes in serum triglyceride concentration following PFOS exposure (Fig. 4-C). PFOS-treated RD mice had lower levels of serum cholesterol, HDL-ch and LDL-ch, which was more significant in 20 mg/kg dose group (Fig. 4-D, E and F). A dose-dependent decrease in serum cholesterol occurred in HFD-fed mice with PFOS exposure (Fig. 4-D), so as the serum HDL-ch (Fig. 4-E). Though not so significant, slight decrease in LDL-ch concentrations was also detected in PFOS-treated HFD individuals, this tended to be more obvious in 20 mg/kg dose group (Fig. 4-F).
Alterations in serum hormone levels
Comparing the median, HFD controls had lower testosterone level (4.54 ng/mL) than RD controls (6.06 ng/mL). PFOS exposure also reduced the average level of testosterone in both RD and HFD-fed mice. However, due to the great fluctuation in the parallel individuals, serum testosterone concentrations did not differ between those PFOS-treated and control mice (Fig. 5). A HFD caused slightly increase in serum estradiol level, 53 pg/mL compared to 34 pg/mL of RD controls (Fig. 5). PFOS exposure had no significant effect on serum estradiol levels of RD-fed mice, but a moderate decrease in HFD ones (Fig. 5).
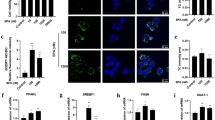
Expression of PPARα and lipid metabolism related genes in liver
PPARα controls the transcriptional expression of key enzymes that are involved in FA uptake and β-oxidation. Feeding extra fat resulted in greater PPARα gene expression in control mice, but not so significant (Fig. 6). Expression of PPARα was not up-regulated by 5 mg/kg or 20 mg/kg PFOS in livers of RD-fed mice. However, this expression was inhibited in HFD-fed individuals, in a dose-dependent manner, turned out to be very significant in 20 mg/kg dose group (P < 0.01, Fig. 6).
Carnitinepalmitoyl transferase 1A (CPT1A), responsible for transportation of fatty acids into the mitochondria and its catabolism; cholesterol 7α-hydroxylase (CYP7A1), key gene in the transformation of cholesterol into bile acids, their expressions were further investigated. Adding more fat in diet caused activation in CPT1A and CYP7A1 expression (P < 0.01, Fig. 7). When feeding a RD, CPT1A expression was up-regulated by 5 and 20 mg/kg PFOS (P < 0.01); 5 mg/kg PFOS exposure also caused slight increase in CYP7A1 expression, while no change occurred after 20 mg/kg PFOS treatment. Nonetheless, this trend was reversed by a HFD following PFOS exposure (Fig. 7). Expression of CPT1A gene was inhibited in HFD-fed mice, displaying a dose-dependent effect (P < 0.01, Fig. 7). CYP7A1 gene expression was inhibited significantly in those mice, P < 0.01 and P < 0.05 for 5 and 20 mg/kg dose group respectively (Fig. 7).
Discussion
PFOS has been considered to disturb lipid metabolism in rodents and human because of its similar structure to fatty acid33,34,35. 5 mg/kg PFOS here did not cause significant change in body weight or food consumption, but more lipids were accumulated in the livers. These mice also had lower serum cholesterol level, as well as the HDL and LDL. However, serum triglyceride level was elevated, indicating the ventral fat store might be released. Increasing serum albumin levels might also contribute to the released triglyceride and fatty acid. We also detected declined liver glycogen storage, which suggested sustaining the serum glucose levels. With increasing dose of PFOS, severe body weight loss was observed. The decreasing liver glycogen storage was not sufficient to release glucose, leading to the reduction in serum glucose levels. Serum triglyceride and cholesterol concentrations were also decreased after 20 mg/kg PFOS exposure, accompanied by much lower levels of HDL and LDL. Additionally, more lipids accumulated in the livers following 20 mg/kg PFOS treatment while the ventral fat index reduced sharply. The accumulation of lipid droplets within hepatic cells was further proved by pathological and ultrastructural assessments. We also examined spleen and thymus cells but found no lipid accumulation36. These results suggest that PFOS exposure disturbed homeostasis of lipid metabolism in BALB/c mice and caused declined liver glycogen storage as well as serum glucose levels.
Besides a RD feeding, here extra fat were added to the feed during PFOS exposure, which is different from former PFOS-related studies. It was reported previously that increasing body weight, hyperglycemia and steatohepatitis occurred in HFD-fed rodents31,32. Similarly, the liver fat content of HFD-fed mice here was significantly elevated, as were serum glucose, cholesterol, HDL and LDL concentrations. Expression of key genes related to lipid metabolism such as CPT1A and CYP7A1 were also up-regulated in the livers. But unlike the control mice, the PFOS-treated HFD mice showed lipid accumulated livers along with decreased serum glucose and lipid levels. Those results were also consistent with performance of RD-fed mice. Therefore, supplement of dietary fat could not moderate the disturbances of PFOS on lipid metabolism.
Molecular mechanism of PFOS-caused toxicities remains unclear. Major opinions focused on the activation of PPARα37,38. Some researchers have demonstrated that PFOS elicited its hepatotoxicity by causing increased expression of PPARα-regulated genes39. However, one study done by Abbott showed that PPARα knockout mice also got developmental toxicity and enlarged livers following PFOS exposure, which occurred in wild type mice40,41. In our study, a HFD indeed induced a slight increase in PPARα expression of control mice. But unlike previous finding39, PFOS caused no stimulation of PPARα gene expression in RD-fed mice while inhibited its expression in HFD-fed individuals. Nevertheless, both RD and HFD-fed mice displayed similar differences in body weight, liver index and lipid accumulation with PFOS exposure; Those mice also got similar fluctuating trend in the serum lipid and lipoprotein levels. Moreover, PFOS altered the expression of CPT1A and CYP7A1 gene,in a different pattern from PPARα. CPT1A and CYP7A1 have been reported to have PPRE response elements in their promoter regions, indicating that they are possibly regulated by PPARα42. We thus consider activation of PPARα might not be the main cause of PFOS-induced changes in lipid metabolism. Due to their key roles in regulating the metabolism, the alteration of CPT1A and CYP7A1 expression might indicate that transportation of fatty acids into the mitochondria and transformation of cholesterol into bile acids was blocked by PFOS. Therefore, the rate of lipid catabolism would be decreased. This may directly contribute to the lipid accumulation in PFOS-exposed livers.
Increased serum albumin level was observed here in PFOS-treated mice, which ascended with the exposure doses. Serum albumin was reported to play an important role in maintaining colloid osmotic blood pressure and transport, distribution of various molecules including fatty acids43,44. The increasing fatty acids released from the ventral fat might be the cause of elevated albumin level here. Since albumin is originally synthesized in the liver, its normal function indicated that the liver remained undamaged. We thus infer that accumulation of lipid in livers of PFOS-treated mice might not be caused by hepatic injury, the disturbance in transport and metabolism of lipids should account for it.
Particularly, 20 mg/kg PFOS-exposed mice got reduced serum glucose level in contrast to their lipid-accumulated livers. This decline might cause an acute increase in energy demand by selective oxidation of liver glycogen, following the decreased liver glycogen content. As it went on, the gluconeogenesis pathway would be activated, including gluconeogenesis from glycerol43. Additionally, less exogenous fat might be ingested and then more glycerol should be mobilized from ventral fat to supply glucose, accompanied by release of fatty acids43. As a result, more fatty acids might be transported by albumin to the liver, while the ventral fat atrophied. Similar phenomenon was observed in our research on PFOA, mice treated with increasing doses of PFOA got reduced serum glucose levels while lipid-accumulated livers43. However, mechanism of this phenomenon as well as its relationship with the disturbed lipid homeostasis needs to be explored.
Plasma lipoproteins mediate the transport and delivery of triglyceride, cholesterol and certain lipid-soluble vitamins to body tissues46,47. HDL participates in reverse cholesterol transport from peripheral tissues to the liver; LDL, revolves from very low density lipoproteins (VLDL), carries endogenous lipids (including triglyceride and cholesterol) from the liver to peripheral tissues for their subsequent processing, storage or secretion46,47. A HFD here significantly elevated serum HDL and LDL levels, aiming to transport the extra fat. Nevertheless, both RD and HFD-fed mice got reduced HDL and LDL levels after PFOS exposure. We previously reported similar results with PFOA-exposed BALB/c mice, but lipid droplets occurred in the hepatocyte nuclei of those mice45. However, considering their structural resemblance, PFOS may act like PFOA in disturbing VLDL synthesis and excretion. As a result, the secretion and normal function of LDL would be also blocked, this further might lead to reduced serum LDL levels. According to this hypothesis, as more lipids were released from ventral fat following PFOS exposure while lipids exportation from livers was blocked, then lipid accumulated-livers and atrophic ventral fat would occur.
In conclusion, a diet high in fat cannot relieve the lipid metabolism disturbance caused by PFOS exposure. We consider the PFOS-caused toxicities were probably irrelevant to PPARα gene expression. As triglyceride in ventral fat were mobilized for gluconeogenesis following decreased serum glucose levels, more free fatty acids might be transported to the liver by albumin; By inhibiting the secretion and normal function of LDL, PFOS could possibly block the exportation of lipids from the liver to peripheral tissues. As a result, more lipids accumulated in the liver but ventral fat store was decreased, serum triglyceride and cholesterol levels were also remarkably reduced. Nevertheless, further researches are required to demonstrate the hypothesis of those mechanisms.
Methods
Animals and Diet
Male BALB/c mice aged 4–5 weeks old were purchased from the Hubei Laboratory Animal Research Center (Hubei, China). Mice at this age gain body weight continuously and lipid metabolism is the most vigorous. The mice were housed in a climate-controlled facility with a temperature of 24 ± 2°C, relative humidity of 60–70% and a 12:12 hr light: dark cycle. Following acclimatization for one week, they were randomly divided into six groups (A, B, C, D, E and F), 8 individuals for each group and housed 4 per cage. During PFOS exposure, mice in groups A, B and C were fed the RD, whereas mice in groups D, E and F were fed a HFD containing 10% more lard and 3% more cholesterol than the RD. The feed was purchased from Beijing HFK Bio-Technology Co., LTD.
Experimental protocol
This experiment was repeated twice for its reliability. The animal treatment was approved by the Ethics Committee of Jianghan University (Wuhan, China). The methods were carried out in accordance with the approved guidelines. PFOS (CAS number 2795-39-3, purity >98%, Sigma-Aldrich, St Louis, MO, USA) was dissolved in 0.5% Tween 20 (Sigma-Aldrich) and given via daily oral gavage to mice for 14 days at doses of 5 mg/kg (groups B and E) and 20 mg/kg (groups C and F). Control mice (groups A and D) received 0.5% Tween 20 without PFOS. The two doses were chosen by referring previous research on PFOS-exposed mice11,12,13 and comparable PFOS concentrations (more than 10 μg/mL) have also been detected in occupational populations7,8. During the exposure, body weight and feed consumption were measured every 2 days and the dose of PFOS was adjusted to the changing body weight every 4 days.
Blood samples were collected by retro-orbital bleeding into sterile polystyrene tubes and the mice were then sacrificed by cervical dislocation. The livers and ventral fat pad were quickly harvested and weighed. Organ indices were calculated as [organ weight (g)/body weight (g)] × 100. Organ samples were fixed immediately either in 4% paraformaldehyde or 2.5% glutaraldehyde for later histopathological or ultrastructural assessment. Additional liver samples were stored at −80°C for following detections.
Detection of serum biochemical parameters and hormone levels
The blood samples were centrifuged at 3000 rpm for 10 min to separate serum for biochemical analysis. Serum albumin, glucose, cholesterol, triglyceride, HDL-ch and LDL-ch were measured using an Abbott Aeroset automated instrument analyzer (Abbott Diagnostics, Abbott Park, Illinois, USA). LDL-ch and HDL-ch were detected as cholesterol content in serum lipoprotein molecules and are regarded here as representative of serum LDL and HDL levels. Serum concentrations of estradiol and testosterone were also determined using an Abbott AXSYM System automated immunoassay analyzer (Abbott Diagnostics, Abbott Park, Illinois, USA).
Determination of liver fat content
Liver fat content was determined as follows: (1) Fresh tissue wrapped in aluminum foil was freeze-dried in an Alpha1-2LD plus freeze dryer (Christ, Osterode am Harz, Germany) for two days. (2) The dried tissue was ground, weighed and denoted as M1. Samples were then mixed with 15 ganhydrous sodium sulfate and extraction was performed using an ASE300 accelerated solvent extractor (Dionex, Sunnyvale, CA, USA) set to the following parameters: solvent dichloromethane/n-hexane (1:1 v/v), pressure 15 M Pa, temperature 150°C. (3) The extractant solution was then transferred into a pre-weighed dry flask (flask weight = W1) and dried using a Laborota 4002 rotary evaporator (Heidolph, Schwabach, Germany). (4) The extract was further dried under a gentle stream of nitrogen and the flask was reweighed (weight of flask + extract = W2). The final dry weight of the fat (M2) was calculated as the difference between W2 and W1. Liver fat content was calculated as M2/M1 × 100%.
Measurement of hepatic glycogen content
Weighed liver samples were homogenized in normal saline. Hepatic glycogen levels were then measured using a hepatic/muscle glycogen detecting assay kit from the Nanking Jiancheng bio-engineering research institute (Nanking, China).
Histopathological observation
Following overnight fixation in 4% paraformaldehyde, the livers were dehydrated by stepwise transfer into increasing concentrations of ethanol (70%, 80%, 85%, 90%, 95% and 100%) and embedded in paraffin. Embedded tissues were sliced into 4 μm sections using a Leica automatic microtome (Leica Microsystems, Wetzlar, Germany) and stained with hematoxylin and eosin (H & E) for histological assessment under light microscopy (Olympus BX41, Tokyo, Japan).
Transmission electron microscopy examination
Liver samples were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) at 4°C for 24 hr. Samples were then washed twice with phosphate buffer (0.1 M, pH 7.4) and post-fixed for 20 min with 1% osmium tetroxide in 0.1 M phosphate buffer (pH 7.4). After dehydration, samples were embedded in Epon-812 epoxy resin and ultrathin sections were made using a LKB-V ultramicrotome (LKB, Bromma, Sweden). Sections were mounted on copper grids and stained with 2% uranyl acetate in a 1% solution of lead citrate for 30 min. The ultrastructure of the liver samples was visualized using a FEI Tecnai G2 (FEI, Hillsboro, OR, USA) transmission electron microscopy operating at 200 kV. Sections were photographed with a Gatan 832 CCD camera (Gatan, Pleasanton, CA, USA).
Expression of hepatic lipid metabolism genes
Total RNA was isolated from liver samples using Trizol reagent (Invitrogen, Carlsbad, CA, USA). RNA quality was verified by confirming that the optical density 260/280 ratio was between 1.8 and 2.0 and by electrophoresis on a 1% agarose formaldehyde gel. Reverse-transcribed cDNA was obtained using Moloney Murine Leukemia Virus (M-MLV) reverse transcriptase (Promega, Madison, WI, USA). Real-time PCR was performed on a PTC-200 thermal cycler (MJ Research, Watertown, CA, USA) using a SYBR Green Master Mix Reagent Kit (Toyobo, Tokyo, Japan). Threshold cycle (CT) values of PPARα, CPT1A and CYP7A1 were analyzed with Opticon Monitor 3 analysis software (Opticon, Luton, UK). Gene expression levels were normalized to the housekeeping gene β-actin. Relative changes in gene expression were assessed using the 2−ΔΔCT method48. Primers were designed using Primer Premier 5.0 (PREMIER Biosoft International, USA) and listed in Table 2.
Statistical analysis
All data were analyzed using SPSS 16.0 software (SPSS, Chicago, IL, USA). Results were expressed as the mean value ± standard error from at least 4 test experiments. One-way ANOVA was used to determine between-group differences followed by Fisher's least significant difference (LSD) test. P < 0.05 was considered significant, P < 0.01 was considered very significant.
References
Hoff, P. T. et al. Perfluorooctane sulfonic acid in bib (Trisopterus luscus) and plaice (Pleuronectes platessa) from the Western Scheldt and the Belgian North Sea: distribution and biochemical effects. Environ Toxicol Chem 22, 608–614 (2003).
Moriwaki, H., Takata, Y. & Arakawa, R. Concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in vacuum cleaner dust collected in Japanese homes. J Environ Monitor 5, 753–757 (2003).
Wang, Y. et al. Distribution of perfluorooctane sulfonate and other perfluorochemicals in the ambient environment around a manufacturing facility in China. Environ Sci Technol 44, 8062–8067 (2010).
Giesy, J. P. & Kannan, K. Global distribution of perfluorooctane sulfonate in wildlife. Environ Sci Technol 35, 1339–1342 (2001).
Kannan, K. et al. Concentrations of perfluorinated acids in livers of birds from Japan and Korea. Chemosphere 49, 225–231 (2002).
Inoue, K. et al. Perfluorooctane sulfonate (PFOS) and related perfluorinated compounds in human maternal and cord blood samples: assessment of PFOS exposure in a susceptible population during pregnancy. Environ Health Persp 112, 1204 (2004).
Olsen, G. W., Burris, J. M., Burlew, M. M. & Mandel, J. H. Epidemiologic assessment of worker serum perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) concentrations and medical surveillance examinations. J Occup Environ Med 45, (3), 260–270 (2003).
Zhou, Z. et al. Highly Elevated Serum Concentrations of Perfluoroalkyl Substances in Fishery employees from Tangxun Lake, China. Environ Sci Technol 10.1021/es4057467 (2014).
Austin, M. E. et al. Neuroendocrine effects of perfluorooctane sulfonate in rats. Environ Health Persp 111, 1485 (2003).
Cui, L. et al. Studies on the toxicological effects of PFOA and PFOS on rats using histological observation and chemical analysis. Arch Environ Con Tox 56, 338–349 (2009).
Keil, D. E., Mehlmann, T., Butterworth, L. & Peden-Adams, M. M. Gestational exposure to perfluorooctane sulfonate suppresses immune function in B6C3F1 mice. Toxicol Sci 103, 77–85 (2008).
Zheng, L., Dong, G. H., Jin, Y. H. & He, Q. C. Immunotoxic changes associated with a 7-day oral exposure to perfluorooctanesulfonate (PFOS) in adult male C57BL/6 mice. Arch Toxicol 83, 679–689 (2009).
Thibodeaux, J. R. et al. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. I: maternal and prenatal evaluations. Toxicol Sci 74, 369–381 (2003).
Hu, W., Jones, P. D., Celius, T. & Giesy, J. P. Identification of genes responsive to PFOS using gene expression profiling. Environ Toxicol and Phar 19, 57–70 (2005).
Seacat, A. M. et al. Sub-chronic dietary toxicity of potassium perfluorooctanesulfonate in rats. Toxicology 183, 117–131 (2003).
Kennedy, G. L. Increase in mouse liver weight following feeding of ammonium perfluorooctanoate and related fluorochemicals. Toxicol Lett 39, 295–300 (1987).
Goldenthal, E. et al. 90-day subacute rat study. Study No. 137-085, International Research and Development Corp., Mattawan, MI. Available on USEPA Public Docket AR-226 (1978).
Ikeda, T. et al. Induction of cytochrome P-450 and peroxisome proliferation in rat liver by perfluorinated octane sulphonic acid (PFOS). Peroxisomes in Biology and Medicine [304–308] (Springer, 1987).
Bjork, J. A. et al. Perfluorooctane sulfonate-induced changes in fetal rat liver gene expression. Toxicology 251, 8–20 (2008).
Rosen, M. B. et al. Gene expression profiling in the liver and lung of perfluorooctane sulfonate-exposed mouse fetuses: comparison to changes induced by exposure to perfluorooctanoic acid. Reprod Toxicol 27, 278–288 (2009).
Haughom, B. & Spydevold, Ø. The mechanism underlying the hypolipemic effect of perfluorooctanoic acid (PFOA), perfluorooctane sulphonic acid (PFOSA) and clofibric acid. Biochimica et Biophysica Acta (BBA)-Mol Cell Biol L 1128, 65–72 (1992).
Fuentes, S. et al. Interactions in developmental toxicology: concurrent exposure to perfluorooctane sulfonate (PFOS) and stress in pregnant mice.Toxicol Lett. 164, 81–89 (2006).
Chang, S. C. et al. Thyroid hormone status and pituitary function in adult rats given oral doses of perfluorooctanesulfonate (PFOS). Toxicology 243, 330–339 (2008).
Feige, J. N. et al. From molecular action to physiological outputs: peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Prog Lipid Res 45, 120–159 (2006).
Yang, Q. & Li, Y. Roles of PPARs on regulating myocardial energy and lipid homeostasis. J Mol Med 85, 697–706 (2007).
Berthiaume, J. & Wallace, K. B. Perfluorooctanoate and N-ethyl perfluorooctane- sulfonamido ethanol; peroxisome proliferation and mitochondrial biogenesis. Toxicol Lett 129, 23–32 (2002).
Heuvel, J. P. V., Thompson, J. T., Frame, S. R. & Gillies, P. J. Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: a comparison of human, mouse and rat peroxisome proliferator-activated receptor-α,-β, and-γ, liver X receptor-β and retinoid X receptor-α. Toxicol Sci 92, 476–489 (2006).
Takacs, M. L. & Abbott, B. D. Activation of Mouse and Human Peroxisome Proliferator–Activated Receptors (α, β/δ, γ) by Perfluorooctanoic Acid and Perfluorooctane Sulfonate. Toxicol Sci 95, 108–117 (2007).
Chen, Y. M. & Guo, L. H. Fluorescence study on site-specific binding of perfluoroalkyl acids to human serum albumin. Arch Toxicol 83, 255–261 (2009).
Lin, S., Thomas, T., Storlien, L. & Huang, X. Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int J Obesity 24, 639–646 (2000).
Huang, B. W., Chiang, M. T., Yao, H. T. & Chiang, W. The effect of high-fat and high-fructose diets on glucose tolerance and plasma lipid and leptin levels in rats. Diabetes Obes Metab 6, 120–126 (2004).
Carmiel-Haggai, M., Cederbaum, A. I. & Nieto, N. A high-fat diet leads to the progression of non-alcoholic fatty liver disease in obese rats. FASEB J 19, 136–138 (2005).
Luebker, D. J. et al. Interactions of flurochemicals with rat liver fatty acid-binding protein. Toxicology 176, 175–185 (2002).
Lau, C. et al. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci 99, 366–394 (2007).
Fletcher, T. et al. Associations between PFOA, PFOS and changes in the expression of genes involved in cholesterol metabolism in humans. Environ Int 57, 2–10 (2013).
Wang, Y. et al. Modulation of dietary fat on the toxicological effects in thymus and spleen in BALB/c mice exposed to perfluorooctane sulfonate. Toxicol Lett 204, 174–182 (2011).
Shipley, J. M. et al. Trans-activation of PPARα and induction of PPARα target genes by perfluorooctane-based chemicals. Toxicol Sci 80, 151–160 (2004).
Wolf, C. J. et al. Activation of Mouse and Human Peroxisome Proliferator− Activated Receptor Alpha by Perfluoroalkyl Acids of Different Functional Groups and Chain Lengths. Toxicol Sci 106, 162–171 (2008).
O'Brien, J. M. et al. W. Perfluorooctane sulfonate (PFOS) toxicity in domestic chicken (Gallus gallus domesticus) embryos in the absence of effects on peroxisome proliferator activated receptor alpha (PPARα)-regulated genes. Com Biochem Phys C 149, 524–530 (2009).
Abbott, B. D. et al. Developmental toxicity of perfluorooctane sulfonate (PFOS) is not dependent on expression of peroxisome proliferator activated receptor-alpha (PPARα) in the mouse. Reprod Toxicol 27, 258–265 (2009).
Qazi, M. R. et al. The atrophy and changes in the cellular compositions of the thymus and spleen observed in mice subjected to short-term exposure to perfluorooctanesulfonate are high-dose phenomena mediated in part by peroxisome proliferator-activated receptor-alpha (PPARα). Toxicology 260, 68–76 (2009).
Mandard, S., Müller, M. & Kersten, S. Peroxisome proliferator-activated receptor α target genes. Cell Mol Life Sci 61, (4), 393–416 (2004).
Nelson, D. L. & Cox, M. M. Lehninger Principles of Biochemistry. [Ahr, K. (ed.)] [551–558, 648–650] (Freeman, W. H. New York, 2008).
He, X. M. & Carter, D. C. Atomic structure and chemistry of human serum albumin. Nature 358, 209–215(1992).
Wang, L. et al. Specific Accumulation of Lipid Droplets in Hepatocyte Nuclei of PFOA-exposed BALB/c Mice. Sci Rep 3, 2174; 10.1038/srep02174 (2013).
Eisenberg, S. Metabolism of apolipoproteins and lipoproteins. Curr Opin Lipidol 1, 205–215 (1990).
Johnson, W. J., Mahlberg, F. H., Rothblat, G. H. & Phillips, M. C. Cholesterol transport between cells and high-density lipoproteins. Biochimica et Biophysica Acta (BBA)-Mol Cell Biol L 1085, 273–298 (1991).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 (2001).
Acknowledgements
The authors wish to thank the National Natural Science Foundation of China (grant numbers 21277062, 21277164 and 21107128) and the Program for New Century Excellent Talents in University (NCET-11-0964) for providing funding for this study.
Author information
Authors and Affiliations
Contributions
Y.L. conceived the idea and designed the research with L.W. L.W., Y.W., Y.L. and J.L. carried out the experiment. L.W. analyzed the data and wrote the manuscript. Y.C.L. and J.Z. supplied theoretical background for the explanation. All the authors contributed to discussion of the results. J.J.F. and A.Q.Z. revised the manuscript. G.B.J. supervised the project.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported license. The images in this article are included in the article's Creative Commons license, unless indicated otherwise in the image credit; if the image is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the image. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Wang, L., Wang, Y., Liang, Y. et al. PFOS induced lipid metabolism disturbances in BALB/c mice through inhibition of low density lipoproteins excretion. Sci Rep 4, 4582 (2014). https://doi.org/10.1038/srep04582
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep04582
This article is cited by
-
A review of cardiovascular effects and underlying mechanisms of legacy and emerging per- and polyfluoroalkyl substances (PFAS)
Archives of Toxicology (2023)
-
Efficient workflow for suspect screening analysis to characterize novel and legacy per- and polyfluoroalkyl substances (PFAS) in biosolids
Analytical and Bioanalytical Chemistry (2022)
-
Exploration of the developmental toxicity of TCS and PFOS to zebrafish embryos by whole-genome gene expression analyses
Environmental Science and Pollution Research (2021)
-
Metabolic disturbance in hippocampus and liver of mice: A primary response to imidacloprid exposure
Scientific Reports (2020)
-
Environmental lipidomics: understanding the response of organisms and ecosystems to a changing world
Metabolomics (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.