Abstract
Osteochondral injuries remain difficult to repair. We developed a novel photo-cross-linkable furfurylamine-conjugated gelatin (gelatin-FA). Gelatin-FA was rapidly cross-linked by visible light with Rose Bengal, a light sensitizer and was kept gelled for 3 weeks submerged in saline at 37°C. When bone marrow-derived stromal cells (BMSCs) were suspended in gelatin-FA with 0.05% Rose Bengal, approximately 87% of the cells were viable in the hydrogel at 24 h after photo-cross-linking and the chondrogenic differentiation of BMSCs was maintained for up to 3 weeks. BMP4 fusion protein with a collagen binding domain (CBD) was retained in the hydrogels at higher levels than unmodified BMP4. Gelatin-FA was subsequently employed as a scaffold for BMSCs and CBD-BMP4 in a rabbit osteochondral defect model. In both cases, the defect was repaired with articular cartilage-like tissue and regenerated subchondral bone. This novel, photo-cross-linkable gelatin appears to be a promising scaffold for the treatment of osteochondral injury.
Similar content being viewed by others
Introduction
Management and repair of osteochondral injuries in the knee remain challenging. Osteochondral injuries involve damage to both the articular cartilage and the underlying subchondral bone. Articular cartilage (hyaline cartilage) has limited intrinsic healing capabilities. Once injured, lesions are typically replaced by fibrocartilage, a scar tissue with different biomechanical properties and impaired mechanical function1,2. Covering the load-bearing surfaces of joint bones with fibrocartilage fails to protect the subchondral bone from further degeneration. As osteochondral defects are often associated with joint mechanical instability, the potential problems after osteochondral injury include early osteoarthritis and/or osteoarthrosis (OA)3. The goals for treatment of osteochondral injuries are to restore hyaline cartilage and subchondral bone regeneration.
Over the last few decades, progress has been made using surgical repair interventions. Recent advances in these treatment methods include the use of tissue specific cells, progenitor cells and appropriate cellular stimulation with growth factors at the affected sites4,5. Currently, tissue-engineering approaches using scaffold-based procedures have been attracting increasing attention6. A scaffold provides an environment for cell attachment, proliferation and differentiation. In addition, scaffolds can be used to achieve drug delivery with high-loading efficiency at specific sites. Biologic scaffolds employed to date as natural polymers include alginate, collagen, fibrin, albumin, hyaluronan, platelet-rich plasma and gelatin7.
Gelatin is a very promising material for cell proliferation and tissue regeneration, but has the disadvantage of mechanical weakness. Recently, chemical modification of gelatin has been reported to improve its mechanical properties by cross-linking with visible light8,9,10,11,12. Photo-induced cross-linking or polymerization is a fast and convenient way to produce gels or high-molecular-weight polymers. The scaffold can be formed at specific sites using injectable solutions and visible light, making the application to a designated site simple and minimally invasive. We have previously developed a new type of gelatin by incorporating furfuryl isocyanate (gelatin-FI), which was photo-cross-linked with Rose Bengal (RB), a food dye. The photo-crosslinkable gelatin-FI was useful as a direct pulp capping material in the dental field13.
Tissue cells feel and respond to the stiffness of their substrate14. The physical properties of scaffold influence cell function and tissue morphogenesis15. Consequently, physical characteristics must be considered when designing hydrogels for tissue-engineering applications16. It was found that the stiffness of the hydrogel strongly affected the cell attachment, focal adhesion, migration and proliferation17. Here, we developed another new gelatin derivative that was modified with furfurylamine (gelatin-FA). Gelatin-FA was consolidated by visible light more rapidly with stiffer properties than gelatin-FI in the presence of RB. In the present study, we demonstrate that this novel modified gelatin-FA is an effective scaffold for osteochondral repair in a rabbit osteochondral defect model. Gelatin-FA hydrogels were used to deliver bone marrow stromal cells (BMSCs) and bone morphogenetic protein-4 (BMP4) with collagen binding domains (CBD) to the affected sites. The results suggest that this modified gelatin, in combination with cells and collagen-binding growth factors, is a promising scaffold for tissue engineering because uniform cell seeding is easy to achieve; the implanted cells survive and differentiate; and growth factor(s) with CBD are retained in the hydrogel.
Results
Properties of gelatin-FA
After visible light irradiation, the mixture was transformed from solution to gel by a photo-oxidation crosslinking (POC) mechanism. The chemistry of gelatin-FA is shown in Fig. 1a. By utilizing the carboxy groups in the gelatin for coupling with furan, the remained amino groups in the gelatin were expected to contribute the adhesiveness and stiffness after crosslinking. The 1H-NMR spectra of gelatin before and after furfurylamine conjugation are shown in Figure 1b. In unconjugated gelatin, there was a broad peak at 7.25 ppm, contributed mostly by the benzene proton of phenylalanine residues. In addition to this peak, gelatin-FA and gelatin-FI had three new peaks found at 6.1, 6.2 and 7.3 ppm, attributed to the furan group. The approximate composition of aromatic amino acids in porcine gelatin is 2%, which is similar to the estimate for furan groups in gelatin-FA (2.9%) based on the peak area in 1H-NMR spectra. To compare gelatin-FA with our previously reported gelatin-FI13, 10% aqueous solutions of gelatin-FA and gelatin-FI were mixed with RB (0.5%) on a plate and illuminated for 10, 30, 60 and 180 s by visible light. The plate was washed to remove uncross-linked solutions and the gelation rate was observed. As shown in Figure 1c, approximately 75% of gelatin-FA formed a hydrogel after 30 s of illumination. Conversely, gelatin-FI remained a solution after 60 s of illumination and only 15% was gelled after 180 s of illumination, indicating that gelatin-FA has more rapid cross-linking.
Properties of modified gelatin.
(a) Synthetic scheme of modified gelatin and the cross-linking mechanism. (b) 1H-NMR spectra of gelatin in D2O before and after modification. (c) Time course of gel formation of furfuryl-conjugated gelatin (gelatin-FA and gelatin-FI, each 10%) with Rose Bengal (0.5%) after visible light illumination.
Next, the rheological properties of the modified gelatin were examined after illumination with visible light. Both the storage (G′) and loss (G″) moduli of gelatin-FA hydrogels were higher than the moduli of gelatin-FI hydrogels, suggesting that gelatin-FA hydrogels had higher elasticity and flexibility (Fig. 2). It has demonstrated that hydrogel with higher G′ showed significantly up-regulated expressions of osteocalcin and runt-related transcription factor 2 (RUNX2), with the presence of alkaline phosphatase and the evidence of calcium accumulation15. Meanwhile, the G″/G′ (equivalent to tan δ) ratio of gelatin-FA hydrogels was relatively low, indicating a low viscosity similar to gelatin-FI hydrogels (Fig. 2). These data suggest that gelatin-FA has better appropriate physical properties relative to gelatin-FI.
Cytotoxicity and phototoxicity
The RB cytotoxicity and phototoxicity toward BMSCs were investigated. The data assessed at 24 h were shown in Figure 3a. RB was cytotoxic to more than 70% of the cells at 1% RB. At 0.1% RB, the percentage of viable cells was significantly improved in the presence of gelatin-FA, indicating a cytoprotection function of gelatin-FA. Gelatin-FA also reduced the phototoxicity by visible light at 0.1% and 0.01% RB concentrations (Fig. 3a). BMSCs in gelatin-FA were kept alive at the same level for 7 days at 0.01% RB with or without light illumination. Even at a higher concentration of RB (0.1%), about 50% BMSCs were alive in gelatin-FA after 7 days whereas all of the cells were dead without gelatin-FA (Fig. 3b). Next, the photo-cross-linkable intensity was examined. To do this, gelatin-FA (15%) with various concentrations of RB (0.005, 0.01, 0.05, 0.1%) was submerged in PBS and exposed to visible light for 2 min. Gelatin-FA hydrogels with 0.05% RB were macroscopically unchanged in PBS for at least 21 days at 37°C (Fig. 3c). RB concentrations below 0.01% failed to form solid gels in the submerged conditions (not shown). The viability of cells in gelatin-FA hydrogels with 0.05% RB was 87.3% on day 1, 55.8% on day 3 and 44.1% on day 7 after light exposure in the submerged conditions (Fig. 3d). The percent viable cells at 24 h in 0.05% RB was larger than those in 0.1% RB (64.0% viable, Fig. 3a). Thus, 0.05% RB was chosen as a suitable concentration for cell-based scaffolds. When BMSCs were cultured in the gelatin-FA hydrogels, DNA and acidic mucopolysaccharide contents were measured. Although the DNA content in the hydrogels decreased with time, the acidic mucopolysaccharide level was unchanged throughout the observation period. The resulting acidic mucopolysaccharide-to-DNA ratio, reflecting chondrogenic differentiation of BMSCs, increased with time for up to 3 weeks, suggesting that BMSCs differentiated into chondrocytes in the gelatin-FA scaffold (Fig. 3e).
Cytotoxicity and phototoxicity.
(a) BMSCs (5 × 103 cells) were cultured in gelatin-FA (15%) and various concentrations of RB (0.01, 0.1 and 1%) and gelled by illumination with visible light for 2 min. After 24 h, the % cell viability was determined. *P < 0.05, **P < 0.001, vs. RB + light illumination (5 wells each, Mann–Whitney U test). (b) BMSCs (5 × 103 cells) were cultured in gelatin-FA (15%) plus RB (0.01 or 0.1%). The % viable cells were monitored at 24 h, 72 h and on day 7 after light illumination with visible light for 2 min. Open circle; RB only, closed circle; RB + gelatin-FA, open square; RB + light illumination, closed square; RB + gelatin-FA + light illumination (5 well each). (c) Gelatin-FA (15%) with 0.05% RB was submerged in PBS and exposed to visible light for 2 min, after which the hydrogels were observed for 21 days at 37°C. Representative photographs (5 wells each) are shown. (d) BMSCs (5 × 103 cells) were suspended in gelatin-FA (15%) with 0.05% RB, submerged in PBS and exposed to visible light for 2 min, after which the hydrogels were observed for 7 days at 37°C. The percent viable cells in the hydrogels were examined at indicated time-points after the light illumination (5 wells each). (e) BMSCs (5 × 104 cells) were suspended in gelatin-FA (15%) and 0.05% RB, submerged in PBS and gelled by illumination with visible light for 2 min. DNA and acidic mucopolysaccharide contents in the gelatin-FA hydrogels were measured at indicated time-points. The resultant acidic mucopolysaccharide-to-DNA ratio was then calculated (5 wells each).
Gelatin-FA as a scaffold for BMSC implantation
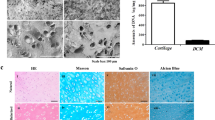
To examine whether gelatin-FA could be useful for tissue engineering, we first employed it as a scaffold for BMSCs. BMSCs (1.5 × 105 cells) were suspended in 15% gelation-FA containing 0.05% RB and the mixtures were implanted into rabbit osteochondral defects and exposed to visible light for 2 min (Fig. 4a). Four weeks later, the defect lesion was filled with regenerated tissues (Fig. 4b). No meaningful differences were found in gelatin-FA-treated groups as compared with the untreated controls at this time point. At 12 weeks, the defect lesion showed degenerative changes in the untreated group. Regenerative tissue filled the defect in the gelatin-FA group, although the surface appeared roughened. In the gelatin-FA plus BMSCs group, the articular surface appeared to be similar to the surrounding intact cartilage (Fig. 4b). The gross grading score demonstrated an improvement in defects after the treatment with gelatin-FA plus BMSCs. Gelatin-FA by itself also improved the repair (Fig. 4c). Histologically, untreated osteochondral defects were not covered with regenerative tissues. Gelatin-FA-treatment allowed granulation tissue to fill the defect at 4 weeks (Fig. 4d). Macrophages were evident in the granulation tissue in gelatin-FA-treatment, which were more prominent in gelatin-FA plus BMSCs (Fig. 4e). Hyaline cartilage-like cells were seen at 12 weeks with their surrounding matrix stained with safranin O (Fig. 4d). The stained area in repaired lesions was significantly larger in the gelatin-FA plus BMSCs group as compared with the gelatin-FA group (Table 1). Histological grading scores at 4 and 12 weeks after surgery revealed that treatment with gelatin-FA plus BMSCs improved tissue regeneration (Fig. 4f). The score at 12 weeks was significantly higher than at 4 weeks (P < 0.01, by Mann–Whitney U test). Gelatin-FA without BMSCs also showed an improved repair compared with untreated controls (Fig. 4f). Type II collagen, not found in untreated groups, was present in the gelatin-FA alone and gelatin-FA plus BMSCs groups and the staining was more intense in the gelatin-FA plus BMSCs group (Fig. 4g). Likewise, aggrecan was not found in untreated groups but stained in gelatin-FA plus BMSCs group (Fig. 4h). Subchondral bone repair was found in the gelatin-FA plus BMSCs group (Fig. 5a). The BV/TV value was significantly higher after treatment with gelatin-FA plus BMSCs than in the other groups (Fig. 5b). All these findings support this modified gelatin-FA as a useful cell scaffold for the treatment of osteochondral defects.
Gelatin-FA as a cell scaffold in an osteochondral defect model.
BMSCs (1.5 × 105 cells) were suspended in gelatin solution (15% gelation-FA + 0.05% RB), implanted into osteochondral defects and exposed to visible light for 2 min. (a) Schematic illustration of the operative procedure. (b) Gross appearance after the procedure. Representative photographs (six femurs each). (c) Gross grading scores at 4 and 12 weeks after the surgery. *P < 0.05, **P < 0.01, ***P < 0.001 (six femurs each). (d) Representative safranin O staining photos at 4 and 12 weeks are shown (six femurs each). The scale bar indicates 500 μm. (e) Representative macrophage staining photos at 4 weeks are shown (six femurs each). The scale bar indicates 200 μm. (f) Histological grading scores at 4 and 12 weeks. *P < 0.05, **P < 0.01, ***P < 0.001 (six femurs each). (g, h) Representative type II collagen immunostaining (g) and aggrecan staining (h) at 12 weeks after the surgery are shown (six femurs each). The scale bar indicates 500 μm (g) and 200 μm (h).
Gelatin-FA as a scaffold for growth factors
We then asked whether gelatin-FA could be used as a scaffold for growth factor-based tissue engineering. We used BMP4 and CBD-BMP4 as growth factors. CBD-BMP4 exhibited stronger and more stable collagen binding activity than did wild-type BMP413,18. Because gelatin is a hydrolyzed form of collagen, we hypothesized that CBD-BMP4 may be better retained in gelatin-FA hydrogels than native BMP4. To test this, BMP4 and CBD-BMP4 (380 nM) were suspended in separate gelatin solutions (15% gelatin-FA and 0.05% RB), photo-cross-linked by visible light and then cultured in PBS for 7 days. The supernatants were collected and BMP4 concentrations measured by ELISA. As shown in Table 2, BMP4 was rapidly released from gelatin-FA hydrogels, whereas CBD-BMP4 was retained in the hydrogels for at least 7 days.
We next implanted BMP4- or CBD-BMP4-containing gelatin solutions (15% gelatin-FA and 0.05% RB) into osteochondral bone defects and exposed them to visible light for 2 min. Growth factor-loaded gelatin-FA hydrogels facilitated cartilage repair (Fig. 6a–d). In particular, CBD-BMP4-loaded gelatin-FA showed complete filling of the defect with a tissue that resembled the surrounding normal hyaline cartilage at 12 weeks (Fig. 6a). Repair tissue in the defects treated with CBD-BMP4-loaded gelatin-FA was strongly stained by safranin O, suggesting the presence of sufficient proteoglycans in the repaired tissue (Fig. 6c). Gross and histological scores showed that CBD-BMP4 led to more cartilage repair than did BMP4. Tissue repair by gelatin-FA itself demonstrated some improvement compared with the untreated group at 12 weeks (Fig. 6b, d). To investigate the molecular mechanisms of repair, the regenerated tissues were harvested at 4 weeks after surgery and the mRNA expression of several chondrogenic factors were examined. SOX9, aggrecan, col1a1 and col2a1 were all expressed at higher levels in the CBD-BMP4-gelatin-FA group than in the other groups, including the BMP4 group (Fig. 6e). Thus, the superior cartilage repair in the CBD-BMP4 group was associated with increased expressions of chondrogenic factors. The BMP4 group failed to increase the expression of these factors at 4 weeks after the surgery, possibly owing to its loss from the hydrogel. Furthermore, CBD-BMP4-loaded gelatin-FA prompted subchondral bone repair (Fig. 7a). The BV/TV value was significantly higher after CBD-BMP4-gelatin-FA treatment than with the other groups at 12 weeks after surgery (Fig. 7b). Therefore, this modified gelatin-FA was also useful as a scaffold for growth factor-based tissue engineering.
Gelatin-FA as a growth-factor scaffold in an osteochondral defect model.
BMP4 or CBD-BMP4 (each 250 nM) was mixed into 15% gelatin-FA containing 0.05% RB and the mixtures were implanted into osteochondral bone defects and exposed to visible light for 2 min. (a) Representative photographs of the gross appearance after the procedure (five femurs each). (b) Gross grading scores at 4 and 12 weeks after the procedure (five femurs each). (c) Representative images of safranin O staining (five femurs each). The scale bar indicates 500 μm. (d) Histological grading scores at 4 and 12 weeks (five femurs each). (e) The expression levels of chondrogenic factors at 4 weeks (5 femurs each). *P < 0.05, **P < 0.01, ***P < 0.001 (five femurs each). ****P < 0.05, vs. untreated control (five femurs each, Mann–Whitney U Test).
Discussion
Scaffolds ideally need to be biocompatible, biodegradable, durable and capable of being formed into desired shapes. The novel modified gelatin-FA developed in the present study appears to be a suitable biomaterial scaffold. Compared with our previously reported gelatin-FI, gelatin-FA is more rapidly cross-linked in the presence of RB by visible-light illumination. Such a rapid consolidation is suitable for clinical use. In addition, gelatin-FA has higher elasticity and flexibility than gelatin-FI, with an equivalent viscosity. These superior properties prompted us to examine the potential of gelatin-FA for use in scaffold-based tissue engineering. By combining gelatin-FA with BMSCs and CBD-BMP4, we demonstrated that photo-cross-linkable gelatin-FA is a promising scaffold for osteochondral repair because it promotes production of a reparative matrix.
Clinical trials have reported the safety and therapeutic effects of BMSCs administration in patients with OA19. The clinical outcomes after treatment with autologous BMSCs were as good as or better than after autologous chondrocyte implantation20. Chondrocyte differentiation from BMSCs in vitro using growth factors requires significant time and expense and subsequent engineered chondrocytes do not fully maintain their chondrogenic properties after in vivo transplantation21. Therefore, employing BMSCs is a reasonable approach for the treatment of osteochondral injuries. One key point when using BMSCs for osteochondral repair is to use an efficient delivery system to localize the cells within a lesion without inhibiting the influx of repair cells from the surrounding tissues. BMSCs are mixtures of autologously acquired pluripotent cells with multilineage differentiation and high proliferation potentials, allowing for their differentiation into osteogenic and chondrogenic lineages22. Studies have demonstrated that the severe anaerobic environment in the joint is efficient for the phenotypic expression of chondrocytes23,24. Maintaining hydration and the anaerobic environment in gelatin-FA hydrogels may stimulate the chondrogenic differentiation of BMSCs. Here, we showed that transplanted BMSCs preserve their chondrogenic differentiation capability in our gelatin-FA scaffold, as determined by the acidic mucopolysaccharide-to-DNA ratio. Alternatively, it is likely that BMSCs also attract host-derived mesenchymal progenitor cells from the subchondral marrow or the adjacent synovium via the release of various cytokines19, which in turn may facilitate osteochondral tissue repair. We demonstrated that regenerated tissue by gelatin-FA plus BMSCs treatment contained more macrophages relative to that by gelatin-FA alone. Further studies are necessary to address the relative contributions of these mechanisms.
The use of growth factors with tissue engineering techniques is another promising therapeutic strategy. To increase the therapeutic potency of gelatin-FA, we employed CBD-BMP4 as a growth factor. CBD-BMP4 is a novel fusion protein that is retained longer than native BMP4 in tissues consisting of collagen and exhibits an exquisite ability to promote in vivo osteogenesis18. BMP4 is capable of inducing osteogenic, as well as chondrogenic, differentiation25,26. Because CBD-BMP4 is retained longer in gelatin-FA hydrogels, we expected it to foster tissue repair. Although both gelatin-FA alone and BMP4-gelatin-FA enhanced tissue repair to some degree, CBD-BMP4-gelatin-FA produced a markedly improved osteochondral repair. During this process, gelatin-FA covering the defect may enhance the integration between the regenerated tissue and the surrounding tissue, supporting tissue repair. The accelerated tissue repair by CBD-BMP4-gelatin-FA appears to be based on endogenous chondrogenic factors expressed by infiltrating cells from the surrounding tissues. SOX9 is a key transcription factor for chondrogenesis27. SOX9 activates genes expressed in proliferating chondrocytes that includes aggrecan28, one of the major structural components of articular cartilage. Col1a1 and col2a1 encode for the major components of collagen types I and II, respectively. The increased expression of these genes in the CBD-BMP4 group suggested that CBD-BMP4 tethering in the hydrogels contributed to the improved tissue repair.
There are other several concerns that were not addressed in this study. Although we succeeded in producing hyaline-like differentiated tissue that integrated with the surrounding native cartilage at 12 weeks after the surgery under full weight bearing without any joint immobilization, the physiological and mechanical properties of the repaired tissue remain to be investigated. Longer-term observation is also necessary. It appears that the transplanted BMSCs not only function as repair cells, but also stimulate the surrounding tissues towards repair. However, the origin of the cells present at the repair site, whether they are the originally transplanted BMSCs or host cells that have migrated to the defect, remains unknown. BMSCs may escape from gelatin-FA hydrogels and move into the surrounding tissues. Although clinical trials have reported the safety of BMSCs19, the multi-potent properties may be potential threats for teratoma/tumorgenesis for a long term. How gelatin-FA hydrogels support tissue repair and when gelatin-FA hydrogels are absorbed and replaced by regenerated tissue is also unclear. We showed that macrophages were present in the regenerated tissue after gelatin-FA treatment. Macrophages are essential to would healing by their interactions with other cellular populations and releasing growth factors29, suggesting that macrophages in the gelatin-FA scaffold facilitate the tissue repair. An interesting question is whether a combination strategy with BMSCs and CBD-BMP4 could increase the regenerative potential of the scaffold, generating an additive or synergistic effect. Further studies are necessary to address these possibilities.
In conclusion, we have developed photo-cross-linkable furfurylamine-conjugated gelatin. With this modified gelatin-FA, tissue-engineering approaches that deliver BMSCs and CBD-BMP4 have been evaluated in a rabbit osteochondral defect model. The results in this study demonstrated that this novel gelatin-FA is useful for cell-based, as well as growth factor-based, scaffolds. Gelatin is a biocompatible, biodegradable and maneuverable material with no harmful effects. Treatment strategies using photo-cross-linkable gelatin-FA could be beneficial for the treatment of osteochondral injuries.
Methods
Materials
Porcine skin gelatin (G2500), furfurylamine (FA), furfuryl isocyanate (FI), deuterium oxide (D2O) and Rose Bengal (RB) were purchased from Sigma-Aldrich (St. Louis, MO, USA). 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) and 2-morpholinoethanesulfonic acid monohydrate (MES) were purchased from Dojindo Molecular Technologies, Inc. (Kumamoto, Japan). N-hydroxysuccinimide (NHS) was purchased from Wako Pure Chemical Industries (Osaka, Japan). Recombinant mouse BMP4, affinity purified anti-mouse BMP4 polyclonal IgG and biotinylated anti-mouse BMP4 IgG were purchased from R&D Systems (Minneapolis, MN, USA). CBD-BMP4 fusion protein was prepared as described previously13.
Synthesis of furfuryl conjugated gelatin
Gelatin (5.0 g) was dissolved in 250 mL MES buffer (50 mM, pH 5.0) at 40°C. Subsequently, 1.533 g (8 mmol) EDC, 0.806 g (7 mmol) NHS and 2.68 g (28 mmol) FA were added and the solution was maintained at 40°C for 24 h. The solution was then dialyzed using a dialysis membrane (molecular weight cut off: 3,500 Da) for 2 days at 40°C. The FA-conjugated gelatin was obtained by lyophilization and referred to as gelatin-FA. Gelatin coupled with furfuryl isocyanate (gelatin-FI) was prepared, as previously reported13. The structure of modified gelatin was characterized using nuclear magnetic resonance spectroscopy (NMR). The samples were dissolved in D2O and the measurement performed using a JNM-AL300 spectrometer (JASCO International Co., Tokyo, Japan).
Visible light induced cross-linking and formation of hydrogels
An aqueous solution of gelatin-FA was mixed with an equal volume of RB. After illuminating for 2 min with visible light from a lamp (Luminar Ace LA-HDF158A, Hayashi Watch-works Co., Tokyo, Japan), the mixture was transformed from a solution to a hydrogel through photo-oxidation cross-linking, as illustrated in Figure 1A. Rheological experiments were carried out with an ARES-G2 rheometer (TA instruments, New Castle, DE, USA) using the parallel plates (25-mm diameter, 0°) configuration at 37°C in the oscillatory mode. The evolution of the storage (G′) and loss (G″) moduli were recorded as a function of time at a frequency of 1 Hz and a strain of 1%.
Animals
Female New Zealand white rabbits weighing 2.0–2.5 kg were obtained from Shimizu Laboratory Supplies (Kyoto, Japan). Animals were housed in a temperature-controlled environment with a 12-h light/12-h dark cycle and allowed free access to water and food. The animal care and use committee at Okayama University approved all animal experiments conducted in this study. All methods were carried out in accordance with the approved guidelines.
Bone marrow stromal cells (BMSCs)
BMSCs were purified as described previously30. In brief, bone marrow cells were aspirated from the humerus, femur and tibia of 5 rabbits. The cells were centrifuged and washed twice with phosphate-buffered saline (PBS, pH 7.4). The cells were suspended in low-glucose Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and antibiotics and cultured overnight in 100 mm × 20 mm dishes at 37°C in a humidified incubator with 5% CO2. Unattached cells were removed and the medium was replaced every 3 days until the cells reached 80% confluence. Colonies formed cells were detached with 0.25% trypsin-EDTA solution, washed twice with medium and divided into two dishes. Cells at passages two to three were used as BMSCs. Under appropriate culture conditions, differentiation assays were performed to confirm cell differentiation into chondrogenic and osteogenic lineages (not shown). The cells were routinely alive more than 97% by trypan blue exclusion.
Cytotoxicity and phototoxicity
RB toxicity and phototoxic effects were examined using BMSCs. BMSCs (5 × 103 cells) were incubated in a 96-well plate at 37°C in a CO2 incubator for 24 h. Gelatin-FA (15%) and RB (0.01, 0.1 and 1%) were added to the DMEM with 1% FBS and gelled by illumination with visible light for 2 min and cultured for 24 h. In other set of experiments, Gelatin-FA (15%) was gelled with RB (0.01 and 0.1%) and exposed to visible light for 2 min and cultured for 24, 72 h and 7 days. Each well was washed three times with PBS and medium was added. The cell proliferation reagent WST-1 (Roche Diagnostics, Indianapolis, IN) was used to determine the number of viable cells according to the manufacturer's instructions. The cell numbers in wells without RB were defined as the controls for each group and the number of cells in each well was normalized to its control to give % viability.
Cell evaluation in the gelatin-hydrogel
BMSCs (5 × 104 cells) were suspended in culture medium (DMEM and 10% FBS) containing 15% gelatin-FA and 0.05% RB. The mixture was cross-linked by visible light illumination for 2 min. The hydrogels were incubated in a culture medium and the medium was replaced every 3 days. On days 1, 7, 14 and 21, the hydrogels were washed twice with PBS and sonicated in 100 μL extraction buffer. The DNA content and acidic mucopolysaccharide content in the hydrogels were determined using DNA Quantity kits (Primary cell Co., Hokkaido, Japan) and acidic mucopolysaccharide assay kits (Primary cell Co.), according to the manufacturers' instructions.
Osteochondral defect model
Rabbits were anesthetized with ketamine (1 mg/kg) and isoflurane (0.25–5 L/min). Cylindrical osteochondral defects were made bilaterally at the center of the medial femoral condyle with a 3.0-mm diameter drill to a depth of 3.0 mm. Forty microliters of gelatin solution (15% gelatin-FA and 0.05% RB) with or without BMSCs or growth factors was applied to the defects and cross-linked with visible light for 2 min. BMP4 and CBD-BMP4 were employed as growth factors. The defects untreated were used as controls. The rabbits employed in BMSCs transplantation model were 18 rabbits (36 femurs: defect control; 6 femurs × 2 time points (4 weeks and 12 weeks), gelatin-FA; 6 femurs × 2, gelatin-FA + BMSCs; 6 femurs × 2). For growth factor transplantation, 20 rabbits were used (40 femurs: defect control; 5 femurs × 2 time points (4 weeks and 12 weeks), gelatin-FA; 5 femurs × 2, gelatin-FA + BMP4; 5 femurs × 2, gelatin-FA + CBD-BMP4; 5 femurs × 2). The femurs were treated at random. The joint capsule and the skin were closed in layers with 5-0 nylon sutures. The rabbits were allowed full weight bearing without any joint immobilization. Rabbits were sacrificed at 4 and 12 weeks after the surgery and the lesion was assessed by a gross grading scheme31. In brief, the defect lesion was scored based on four categories, each on a scale of 0–4 (best score: 16): coverage, neocartilage color, defect margins and surface. The femoral condyle was then resected and scanned by microcomputed tomography (micro-CT; Hitachi Aloka, Tokyo, Japan) at 48-μm slices. Regions of interest were centered on the cylindrical defect area and analyzed by image-analyzing software AZE VirtualPlace (AZE, Ltd, Tokyo, Japan). The bone growth was measured as bone volume (BV) per tissue volume (TV). Subsequently, the femoral condyle was fixed in 4% paraformaldehyde, decalcified in 10% EDTA, embedded in paraffin and the sections were stained with hematoxylin and eosin and safranin O. The sections were assessed by a histological grading scheme31, in which the defect lesion was scored on a scale of matrix (0–4), cell distribution (0–3), surface (0–4), safranin O stain (0–4) and percent safranin O in defect (0–4), with 19 being the best score. For immunohistochemistry, the sections were incubated with a monoclonal antibody to macrophages (DAKO, Glostrup, Denmark), collagen type II (Thermo Fisher Scientific, Waltham, MA), aggrecan (Abcam, Cambridge, UK) or control IgG (5 μg/mL) for 1 h at room temperature. The specimens were rinsed and then incubated for 30 min with peroxidase-labeled polymer (DAKO) at room temperature. Diaminobenzidine (DAKO) was used as a chromogen and counter staining was performed with hematoxylin.
Quantitative real-time polymerase chain reaction (PCR)
Regenerated tissues were homogenized with ISOGEN (Nippon Gene Co., Toyama, Japan) and total RNA was isolated according to the manufacturer's instructions. First-strand cDNA was synthesized from 2 μg of total RNA with oligo (dT)12–18 as primers and the cDNAs were used as a template for PCR. Quantitative real-time PCR was performed with Brilliant III Ultra-Fast SYBR Green QPCR Master Mix (Agilent Technologies, Santa Clara, CA, USA) and specific primers. To validate the SYBR Green PCR products, a dissociation step was performed to verify the Tm (annealing temperature) of the SYBR Green PCR product after the PCR was run. The expression levels of each mRNA were normalized to the expression of the housekeeping gene hypoxanthine phosphoribosyltransferase. The primers used in this study are listed in Supplementary Table S1.
Statistical analysis
Statistical analyses were performed using ANOVAs for multiple samples if not otherwise specified. In some cases, Mann–Whitney U tests were used. A P-value of <0.05 was considered statistically significant.
References
Buckwalter, J. A., Mankin, H. J. & Grodzinsky, A. J. Articular cartilage and osteoarthritis. Instr Course Lect 54, 465–480 (2005).
Mandelbaum, B. R. et al. Articular cartilage lesions of the knee. Am J Sports Med 26, 853–861 (1998).
Buckwalter, J. A., Martin, J. A. & Brown, T. D. Perspectives on chondrocyte mechanobiology and osteoarthritis. Biorheology 43, 603–609 (2006).
Nukavarapu, S. P. & Dorcemus, D. L. Osteochondral tissue engineering: Current strategies and challenges. Biotechnol Adv 31, 706–721 (2013).
Panseri, S. et al. Osteochondral tissue engineering approaches for articular cartilage and subchondral bone regeneration. Knee Surg Sports Traumatol Arthrosc 20, 1182–1191 (2012).
Filardo, G., Kon, E., Roffi, A., Di Martino, A. & Marcacci, M. Scaffold-based repair for cartilage healing: a systematic review and technical note. Arthroscopy 29, 174–186 (2013).
Olson, A., Graver, A. & Grande, D. Scaffolds for articular cartilage repair. J Long Term Eff Med Implants 22, 219–227 (2012).
Hoch, E., Schuh, C., Hirth, T., Tovar, G. E. & Borchers, K. Stiff gelatin hydrogels can be photo-chemically synthesized from low viscous gelatin solutions using molecularly functionalized gelatin with a high degree of methacrylation. J Mater Sci Mater Med 23, 2607–2617 (2012).
Hoshikawa, A. et al. Encapsulation of chondrocytes in photopolymerizable styrenated gelatin for cartilage tissue engineering. Tissue Eng 12, 2333–2341 (2006).
Hu, X., Ma, L., Wang, C. & Gao, C. Gelatin hydrogel prepared by photo-initiated polymerization and loaded with TGF-beta1 for cartilage tissue engineering. Macromol Biosci 9, 1194–1201 (2009).
Lien, S. M., Ko, L. Y. & Huang, T. J. Effect of pore size on ECM secretion and cell growth in gelatin scaffold for articular cartilage tissue engineering. Acta Biomater 5, 670–679 (2009).
Okino, H., Nakayama, Y., Tanaka, M. & Matsuda, T. In situ hydrogelation of photocurable gelatin and drug release. J Biomed Mater Res 59, 233–245 (2002).
Son, T. I. et al. Visible light-induced crosslinkable gelatin. Acta Biomater 6, 4005–4010 (2010).
Discher, D. E., Janmey, P. & Wang, Y. L. Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 (2005).
Wang, L. S., Du, C., Chung, J. E. & Kurisawa, M. Enzymatically cross-linked gelatin-phenol hydrogels with a broader stiffness range for osteogenic differentiation of human mesenchymal stem cells. Acta Biomater 8, 1826–1837 (2012).
Brandl, F., Sommer, F. & Goepferich, A. Rational design of hydrogels for tissue engineering: impact of physical factors on cell behavior. Biomaterials 28, 134–146 (2007).
Wang, L. S., Boulaire, J., Chan, P. P., Chung, J. E. & Kurisawa, M. The role of stiffness of gelatin-hydroxyphenylpropionic acid hydrogels formed by enzyme-mediated crosslinking on the differentiation of human mesenchymal stem cell. Biomaterials 31, 8608–8616 (2010).
Shiozaki, Y. et al. Enhanced in vivo osteogenesis by nanocarrier-fused bone morphogenetic protein-4. Int J Nanomedicine 8, 1349–1360 (2013).
Gupta, P. K., Das, A. K., Chullikana, A. & Majumdar, A. S. Mesenchymal stem cells for cartilage repair in osteoarthritis. Stem Cell Res Ther 3, 25 (2012).
Nejadnik, H., Hui, J. H., Feng Choong, E. P., Tai, B. C. & Lee, E. H. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med 38, 1110–1116 (2010).
Pelttari, K. et al. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum 54, 3254–3266 (2006).
Pittenger, M. F. et al. Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147 (1999).
BASSETT, C. A. & HERRMANN, I. Influence of oxygen concentration and mechanical factors on differentiation of connective tissues in vitro. Nature 190, 460–461 (1961).
Hansen, U. et al. Combination of reduced oxygen tension and intermittent hydrostatic pressure: a useful tool in articular cartilage tissue engineering. J Biomech 34, 941–949 (2001).
Kuroda, R. et al. Cartilage repair using bone morphogenetic protein 4 and muscle-derived stem cells. Arthritis Rheum 54, 433–442 (2006).
Pang, E. K. et al. Effect of recombinant human bone morphogenetic protein-4 dose on bone formation in a rat calvarial defect model. J Periodontol 75, 1364–1370 (2004).
de Crombrugghe, B. et al. Transcriptional mechanisms of chondrocyte differentiation. Matrix Biol 19, 389–394 (2000).
Sekiya, I. et al. SOX9 enhances aggrecan gene promoter/enhancer activity and is up-regulated by retinoic acid in a cartilage-derived cell line, TC6. J Biol Chem 275, 10738–10744 (2000).
Brancato, S. K. & Albina, J. E. Wound macrophages as key regulators of repair: origin, phenotype and function. Am J Pathol 178, 19–25 (2011).
O'oughlin, A. et al. Topical administration of allogeneic mesenchymal stromal cells seeded in a collagen scaffold augments wound healing and increases angiogenesis in the diabetic rabbit ulcer. Diabetes 62, 2588–2594 (2013).
Wayne, J. S., McDowell, C. L., Shields, K. J. & Tuan, R. S. In vivo response of polylactic acid-alginate scaffolds and bone marrow-derived cells for cartilage tissue engineering. Tissue Eng 11, 953–963 (2005).
Acknowledgements
We thank Dr. Takayuki Furumatsu, Ms. Reina Tanaka, Mr. Yasuharu Arashima and Mr. Haruyuki Watanabe for their excellent technical assistance. This work was supported in part by 1) grants from Ministry of Education, Culture, Sports, Science and Technology, Japan and the Japan Society for the Promotion of Science, Grant-in-Aid for Scientific Research (S) of KAKENHI 22220009 and (C) of KAKENHI 25462334 and by 2) the Strategic International Research Cooperative Program, Japan Science and Technology Agency (JST), AS232Z00465F.
Author information
Authors and Affiliations
Contributions
T.M., A.M., T.O. and Y.I., led the project and conducted the data analysis. T.M. and A.M., wrote the paper. T.M., Y.S., K.Y., A.Y., M.K., Y.Y., D.Z., T.K. and M.T., performed the experiments and collected the data.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Dataset 1
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Mazaki, T., Shiozaki, Y., Yamane, K. et al. A novel, visible light-induced, rapidly cross-linkable gelatin scaffold for osteochondral tissue engineering. Sci Rep 4, 4457 (2014). https://doi.org/10.1038/srep04457
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep04457
This article is cited by
-
Colorimetric Sensing Films of Visible-Light Curable Furfuryl Gelatin for Visual Monitoring of Chicken Freshness
Food and Bioprocess Technology (2024)
-
Natural bio-based monomers for biomedical applications: a review
Biomaterials Research (2021)
-
Use of mesenchymal stem cells seeded on the scaffold in articular cartilage repair
Inflammation and Regeneration (2018)
-
Native-mimicking in vitro microenvironment: an elusive and seductive future for tumor modeling and tissue engineering
Journal of Biological Engineering (2018)
-
Collagen-Binding Hepatocyte Growth Factor (HGF) alone or with a Gelatin- furfurylamine Hydrogel Enhances Functional Recovery in Mice after Spinal Cord Injury
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.