Abstract
To evaluate treatment outcomes in children with acute lymphoblastic leukemia (ALL) over the past 3 decades, we assessed the survival of children with ALL in the Surveillance, Epidemiology and End Results (SEER) database. Among 12,096 patients from 18 SEER sites diagnosed from 1981 to 2010, survival rates improved each decade from 74.8% to 84.5% to 88.6% at 5 years and from 69.3% to 80.9% to 85.5% at 10 years (P < 0.0001). For ages 10–14 years, 10-year survival increased by more than 20 percentage points to 75.3%, but for infants, it remained low at 54.7%. Improvements in survival rates were observed in both sexes, but survival rates were higher in girls than in boys. For ages 0–14 years during the 2001–2010 period, the 10-year relative survival rates were 87.8% in girls and 83.6% in boys (P < 0.01). Survival rates in child with ALL are expected to further improve with continuous advance in therapies such as targeted therapy and personalized therapy.
Similar content being viewed by others
Introduction
Acute lymphoblastic leukemia (ALL) is the most common malignancy of childhood, accounting for approximately 25% of all childhood cancers and about 77% of childhood leukemias. An estimated 6,070 new cases (3,350 male patients and 2,720 female) were newly diagnosed in 2013 in the United States1. With improvements in treatment, survival rates of patients with ALL have increased over the past few decades2,3,4,5,6,7,8,9,10,11,12. The National Cancer Institute (NCI) Surveillance, Epidemiology and End Results (SEER) Program reported that the 5-year survival rate for U.S. patients younger than age 15 years with ALL increased from 80.2% in 1990–1994 to 87.5% in 2000–200413. We sought to determine whether this trend in improved survival had continued over the past decade.
To evaluate treatment outcomes in children with ALL over the past 3 decades, we assessed the survival of children with ALL in a large U.S. population data set, the SEER database. In this study, we used period analysis to examine changes in 10-year relative survival probabilities for children diagnosed with ALL before the age of 15 years14.
Results
Incidence of ALL at the 9 original SEER sites
For the incidence study, 5,766 patients aged 0–14 years with a diagnosis of ALL between 1981 and 2010 and no previous cancer diagnosis were identified from the data for the 9 original NCI SEER sites. The number of children diagnosed with ALL increased by more than 25% over the 3 decades (1981–1990, 1991–2000, 2001–2010), from 1,693 to 1,942 to 2,131, respectively; this increase was especially pronounced for the 1–4 years age group: from 860 to 1,029 to 1,118 (Table 1, Supplementary Figure S1a). The percentages of patients in each age group were fairly consistent across the 3 decades. The group aged 1–4 years accounted for approximately 51% of cases in the first decade, 53% in the second decade and 52% in the last decade. Those less than 1 year old accounted for about 3% to 5% of total cases in all 3 decades. More boys than girls aged 0–14 years were diagnosed with ALL; the number increased by 30%, from 921 to 1,197, in boys and by 21%, from 772 to 934, in girls across the 3 decades.
As shown in Table 1 and Supplementary Figure S1b, the incidence of ALL increased across the 3 decades (3.4, 3.5 and 3.7 per 100,000 population, respectively), especially for the 1–4 years age group (6.3, 6.9 and 7.4 per 100,000 population, respectively). In subgroups of children aged 0–14, the incidences were 3.6, 3.7 and 4.1, respectively, for boys and 3.1, 3.1 and 3.3, respectively, for girls per 100,000 population over the 3 decades; the incidences were lower in poor areas than in affluent areas for the first 2 decades, but between 2001 and 2010, the incidence in high-poverty counties increased to 4.3 per 100,000 population, higher than that in low and medium poverty counties, which reversed the previous trend. The high incidences in white children kept rising, to 4.1 per 100,000 population in the last decade, which accounted for most of the overall increase in incidence among children. In contrast, the incidence among black children remained low, 1.9 per 100,000 population, across the 3 decades (Table 1).
Relative survival estimates for 18 SEER sites
The survival analysis results from this large, population-based study over 30 years indicated that the 10-year survival rate has improved in patients from all the age groups analyzed. Overall, 12,096 patients were identified from the data for 18 NCI SEER sites between 1981 and 2010. Data on relative survival and point estimates of the percentages of patients with ALL in each of the 3 periods are provided in Table 2. The relative survival rates (RSRs) increased in patients aged 0–14 years, with an improvement in the 5-year survival rates from 74.8% to 84.5% to 88.6%, respectively, for each successive decade (1981–1990, 1991–2000 and 2001–2010). The findings were similar for 10-year survival rates, which increased by more than 15 percentage points, from 69.3% to 80.9% to 85.5%, respectively (P < 0.0001 between any 2 successive decades for both 5-year and 10-year survival rates).
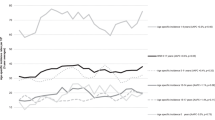
In particular, for children who were 10–14 years old at diagnosis, an age range traditionally associated with a poorer prognosis, the 10-year survival rate increased by more than 20 percentage points: 54.1% to 69.4% to 75.3%, respectively. The largest increase was from the 1981–1990 to the 1991–2000 decade; the survival rate increased more significantly for patients in this age group than for patients in the other age groups.
The trends in overall 10-year survival rates for the 4 age groups over the 3 decades are shown in Figure 1. With each year of follow-up up to 10 years, there was a significant progressive increase in survival in patients in most age groups. These improved outcomes likely are attributable to changes in how ALL is treated, including improved infection control, better supportive care, improved methods for studying cytogenetic and molecular abnormalities and refinement of information about diagnosis and prognosis. The increase in survival was not significant for patients in the age 0 group between 1991–2000 and 2001–2010. Among these infants, the 5-year and 10-year survival rates were still poor, 55.8% and 54.7%, respectively, in the last decade.
Improvements in RSRs were observed in both sexes (Figure 2 & Supplementary Figure S4, Supplementary Tables S1, S2, S3). In most cases, survival estimates were higher in girls than in boys. However, the differences were generally small and the confidence intervals overlapped. The 10-year survival rates were 67.6%, 79.7% and 83.6%, respectively, in boys and 71.4%, 82.5% and 87.8%, respectively, in girls over the 3 decades (P = 0.0059 between boys and girls for 10-year survival rates during 2001–2010).
After exclusion of 4 patients whose county attributes was defined as “blank”, there remained 12,092 patients (99.9%) for the survival analysis of the area socioeconomic status stratification. Improvements in survival were also observed in all 3 area socioeconomic status groups, but the gap in survival between the low poverty and medium poverty groups was widened (Figure 3a & Supplementary Tables S4). The 5-year survival rates increased in the low poverty and medium poverty groups, respectively, from 76.2% and 74.5% in 1981–1990 to 91.7% and 87.1% in 2001–2010. Similarly, the 10-year survival rates for these two groups increased from 70.7% and 68.8% to 89.5% and 83.7%, respectively (P < 0.0001 for both 5-year and 10-year survival rates during 2001–2010). For the 2.8%–8.5% of children who resided in high-poverty counties, the 5-year survival rate increased from 51.1% to 86.4% and the 10-year survival rate increased from 49.0% to 81.4% from the 1981–1990 to the 2001–2010 decade, which reduced the disparity between the high-poverty and the low- and medium-poverty groups.
After exclusion of 92 patients whose race was defined as “unknown”, there remained 12,004 patients (99.2%) for the survival analysis of race stratification. Survival rates increased for all races over the 3 decades studied and the gap in survival between whites and blacks significantly narrowed. The 5-year survival rates for whites and blacks, respectively, increased from 76.3% and 56.6% in 1981–1990 to 88.9% and 86.1% in 2001–2010; similarly, the 10-year survival rates increased from 71.0% and 50.3% to 85.7% and 85.1%, respectively (Figure 3b & Supplementary Table S5).
A more comprehensive picture of survival estimates according to time since diagnosis for children diagnosed with ALL is shown by Kaplan–Meier survival analysis; the resulting survival curves show longer survival trends (30 years and 20 years) for the 1981–1990 and 1991–2000 groups (Supplementary Figures S2 & S3). Significant improvement in survival was seen for all age groups across the 3 decades and these differences were statistically significant, except for the age 0 group between 1991–2000 and 2001–2010 (p = 0.397).
Discussion
Our analysis showed that, over the 3 decades, from 1981 to 2010, overall RSRs in children aged 0–14 improved each decade from 69.3% to 80.9% to 85.5% at 10 years. For aged 10–14 years, 10-year survival increased by more than 20 percentage points to 75.3%, but for infants, it remained low at 54.7%.
Pulte et al. reported 7 years ago that the projected estimated 5-year survival rate for ALL patients aged 0–14 years13 was 90.6% (88.0%–92.7%) for 2005–2009, which is slightly higher than the current estimate of 88.4% (87.0%–89.8%) with the newest version of SEER data. Considering that 88.4% is still within Pulte et al.'s confidence interval and that estimates of survival tend to underestimate the survival of newly diagnosed patients15, the projected estimates still can be accepted as a valuable aid to clinical judgment. Although projected period estimates of survival are expected to be even more up to date than conventional period estimates, they are statistically less precise15.
Our data showed there is a sex difference in the incidence of childhood ALL. It is well known that boys have higher risks of developing a number of health conditions, including certain cancers. This inherent vulnerability is generally attributed to the greater difficulty in repairing damage to genes on the Y chromosome versus the X or to the epigenetic differences between boys and girls. We also found that the incidence of ALL was lower in poor areas than in affluent areas between 1981 and 2000, but this gap diminished over time and the trend was reversed during the decade of 2001–2010, which partially explains why associations between socioeconomic status and childhood ALL have varied with place, time and study design16,17,18.
Our survival results showed that both boys and girls have benefited from improvements in ALL therapy, but sex differences in prognosis still exist. Although the true cause of the difference is unknown, genetic and epigenetic characteristics may play a role in response of leukemic cells to therapy.
Area socioeconomic status is affected by complex influences, including social support, environment, health care accessibility and community culture and it has a significant impact on survival. It was very gratifying to find that the survival difference was significantly diminished between the white and black children in our study. Our results indirectly indicate that the influence of race on survival is closely linked with socioeconomic status rather than genetic differences or the bioavailability of drugs used in the treatment of ALL.
The strength of this study is the large sample size representing diverse populations over 3 decades. In addition, population-based registries are a great resource for the analysis of incidence, mortality rates and trends. But several limitations of the study must be considered. First, the data reflect only selected SEER areas that provided data over the 3 decades and may not be fully applicable to other geographic locations that are not part of the SEER registry. Second, even with period analysis, the estimates of survival tend to underestimate the survival of newly diagnosed patients. Third, SEER data do not contain detailed information concerning chemotherapy treatments, toxicity profiles and participation in clinical trials. Therefore, a potential link between survival results and type of treatment cannot be assessed. Finally, the study might have been affected by several sources of error and bias. For example, underregistration or misclassification of cases and variation of socioeconomic status within and among counties may bias estimates19,20.
In view of these data showing cure rates higher than 80% for children with ALL treated in modern centers, many of these children will lead healthy, productive lives as long-term cancer survivors. However, these data do not reflect the complexity, expense and toxic effects of contemporary multiagent treatments. A recent study with up to 30 years of follow-up observed an increased risk of acute non-lymphoblastic leukemia up to 10 years after successful treatment of childhood ALL and an increased risk of solid tumors that continued for up to 30 years after diagnosis. Patients who are successfully treated for childhood ALL are at increased risk for a number of serious health problems and require close monitoring of their health throughout life21,22.
The increase in survival was still not significant for infant patients. Infant ALL is usually associated with MLL gene rearrangement; by contrast, the incidence of non-MLL-rearranged B lymphoblastic leukemia peaks between 2 and 5 years (aged 1–4 years group)23. Thus, the different intrinsic mechanisms may affect the prognosis of patients in the 2 age groups.
In conclusion, our data demonstrate a marked improvement in survival among pediatric ALL patients over the last 3 decades that is most likely due to improvements in therapy. However, the survival rate in infant ALL remains low. A better understanding of the mechanisms and regulation of molecular interactions should enable the design of novel targeted therapies for these children. In the future, further improvements in survival may be observed as personalized novel and effective therapeutics with tolerable side effect profiles are developed.
Methods
The data analyzed in the present study were obtained from the SEER Program, which was created in 1973 to collect cancer statistics in the United States. The SEER Program collects and distributes high-quality, comprehensive cancer data from a number of population-based cancer registries. The SEER Program registries routinely collect data on patient demographics, primary tumor site, tumor morphology and stage at diagnosis, first course of treatment and follow-up for vital status (through linkage with the National Center for Health Statistics). Quality control has been an integral part of SEER since its inception. The SEER population is similar to the U.S. population in terms of education and socioeconomic levels but has a slightly greater proportion of urban and foreign-born individuals among both the children and adults. SEER coverage includes 25% of White Americans, 26% of African Americans, 38% of Hispanics, 44% of American Indians and Alaska Natives, 50% of Asians and 67% of Hawaiian/Pacific Islanders24.
Since 1973, the SEER registry has grown through the addition of new sites. For the assessment of cancer incidence, to ensure consistency across the 3 decades studied, we obtained data only for patients registered at the original 9 SEER sites: the states of Connecticut, Iowa, New Mexico, Utah and Hawaii and the metropolitan areas of Atlanta, Detroit, San Francisco-Oakland and Seattle-Puget Sound, which cover regions accounting for approximately 10% of the U.S. population. However, for our cancer survival analysis, to get more reliable results, we obtained data for patients registered at 18 SEER sites: the original 9 sites plus Los Angeles, San Jose-Monterey, Rural Georgia, the Alaska Native Tumor Registry, Greater California, Kentucky, Louisiana, New Jersey and Greater Georgia which account for a larger population (approximately 28% of the U.S. population).
For this analysis, we included patients who were younger than 15 years at first diagnosis of ALL, had no prior malignancy and were followed up for vital status through December 31, 2010. Patients whose cancer was diagnosed by autopsy or reported only on a death certificate were excluded. Leukemia diagnoses were identified using the criteria established by the World Health Organization International Classification of Diseases for Oncology. Data obtained included the incidence and RSRs for patients diagnosed with ALL for the periods from 1981 to 1990, from 1991 to 2000 and from 2001 to 2010. Analyses were further stratified by the children's sex, socioeconomic status, race (White, Black and Other) and age at diagnosis (0 year [less than 1 year], 1–4 years, 5–9 years and 10–14 years). Area socioeconomic status was determined by the county poverty rate19,20, which is the percentage of persons in the county living below the national poverty threshold in Census 2000. The county poverty rates in this report were categorized into three levels using the same cut points as the NCI monograph25: <10% (low poverty areas), 10–19.99% (medium poverty areas) and ≥20% (high poverty areas). For incidence and survival rate calculations, SEER Program recoded detailed race information into four major categories: White, Black, Other (American Indian/AK Native, Asian/Pacific Islander) and Unknown. Patients whose race or county level poverty rate was defined as “unknown” or “blank” were excluded during the stratification analysis.
Incidence rates were expressed per 100,000 population and age adjusted to the 2000 U.S. standard population. Cancer patient survival is typically measured from the date of diagnosis to the date of death. Period analysis methodology was applied to calculate the RSR, which measures mortality attributable, either directly or indirectly, to leukemia. The RSR is calculated as the ratio of the absolute survival rate of leukemia patients divided by the expected survival rate for a group of individuals of the corresponding age, sex and race in the general population26.
This analysis was designed to illustrate the trends in patient outcomes over time. Relative survival point estimates (expressed as percentages), mean ± standard error, were calculated in SEER*Stat software (available at: seer.cancer.gov/seerstat) according to standard statistical methodology using the Elderer II method27. Differences in relative survival between each calendar period were also calculated. Kaplan–Meier curves were constructed to estimate overall survival and differences between the curves were assessed using the 2-tailed log-rank test. STATA 12.0 software (Stata Corporation, College Station, TX, USA) was used for analyses. A 2-tailed test P value < 0.01 was accepted as statistically significant for the large sample analyses.
References
Siegel, R., Naishadham, D. & Jemal, A. Cancer statistics, 2013. CA Cancer J Clin 63, 11–30 (2013).
Hunger, S. P. et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the Children's Oncology Group. J Clin Oncol 30, 1663–1669 (2012).
Pui, C. H. et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med 360, 2730–2741 (2009).
Veerman, A. J. et al. Dexamethasone-based therapy for childhood acute lymphoblastic leukaemia: results of the prospective Dutch Childhood Oncology Group (DCOG) protocol ALL-9 (1997–2004). Lancet Oncol 10, 957–966 (2009).
Salzer, W. L. et al. Long-term results of the pediatric oncology group studies for childhood acute lymphoblastic leukemia 1984–2001: a report from the Children's Oncology Group. Leukemia 24, 355–370 (2010).
Gaynon, P. S. et al. Long-term results of the children's cancer group studies for childhood acute lymphoblastic leukemia 1983–2002: a Children's Oncology Group Report. Leukemia 24, 285–297 (2010).
Pui, C. H. et al. Long-term results of St Jude Total Therapy Studies 11, 12, 13A, 13B and 14 for childhood acute lymphoblastic leukemia. Leukemia 24, 371–382 (2010).
Moricke, A. et al. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia 24, 265–284 (2010).
Silverman, L. B. et al. Long-term results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985–2000). Leukemia 24, 320–334 (2010).
Conter, V. et al. Long-term results of the Italian Association of Pediatric Hematology and Oncology (AIEOP) Studies 82, 87, 88, 91 and 95 for childhood acute lymphoblastic leukemia. Leukemia 24, 255–264 (2010).
Kamps, W. A. et al. Long-term results of Dutch Childhood Oncology Group studies for children with acute lymphoblastic leukemia from 1984 to 2004. Leukemia 24, 309–319 (2010).
Mitchell, C., Richards, S., Harrison, C. J. & Eden, T. Long-term follow-up of the United Kingdom medical research council protocols for childhood acute lymphoblastic leukaemia, 1980–2001. Leukemia 24, 406–418 (2010).
Pulte, D., Gondos, A. & Brenner, H. Trends in 5- and 10-year survival after diagnosis with childhood hematologic malignancies in the United States, 1990–2004. J Natl Cancer Inst 100, 1301–1309 (2008).
Brenner, H. & Hakulinen, T. Up-to-date and precise estimates of cancer patient survival: model-based period analysis. Am J Epidemiol 164, 689–696 (2006).
Brenner, H. & Hakulinen, T. Up-to-date estimates of cancer patient survival even with common latency in cancer registration. Cancer Epidemiol Biomarkers Prev 15, 1727–1732 (2006).
Kroll, M. E., Stiller, C. A., Murphy, M. F. & Carpenter, L. M. Childhood leukaemia and socioeconomic status in England and Wales 1976–2005: evidence of higher incidence in relatively affluent communities persists over time. Br J Cancer 105, 1783–1787 (2011).
Adam, M., Rebholz, C. E., Egger, M., Zwahlen, M. & Kuehni, C. E. Childhood leukaemia and socioeconomic status: what is the evidence? Radiat Prot Dosimetry 132, 246–254 (2008).
Poole, C., Greenland, S., Luetters, C., Kelsey, J. L. & Mezei, G. Socioeconomic status and childhood leukaemia: a review. Int J Epidemiol 35, 370–384 (2006).
Krieger, N., Chen, J. T., Waterman, P. D., Rehkopf, D. H. & Subramanian, S. V. Race/ethnicity, gender and monitoring socioeconomic gradients in health: a comparison of area-based socioeconomic measures--the public health disparities geocoding project. Am J Public Health 93, 1655–1671 (2003).
Krieger, N. et al. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter?: the Public Health Disparities Geocoding Project. Am J Epidemiol 156, 471–482 (2002).
Hijiya, N. et al. Cumulative incidence of secondary neoplasms as a first event after childhood acute lymphoblastic leukemia. JAMA 297, 1207–1215 (2007).
Schmiegelow, K. et al. Second malignant neoplasms after treatment of childhood acute lymphoblastic leukemia. J Clin Oncol 31, 2469–2476 (2013).
Inaba, H., Greaves, M. & Mullighan, C. G. Acute lymphoblastic leukaemia. Lancet 381, 1943–1955 (2013).
Surveillance, Epidemiology and End Results (SEER) Program. Research data (1973–2010), www.seer.cancer.gov. National Cancer Institute, Division of Cancer Control and Population Services, Surveillance Research Program, Surveillance Systems Branch, released April 2013 [accessed Nov 6, 2013].
Singh, G. K., Miller, B. A., Hankey, B. F. & Edwards, B. K. Area Socioeconomic Variations In US Cancer Incidence, Mortality, Stage, Treatment, And Survival, 1975–1999. National Cancer Institute, Bethesda. Report No.: NIH Publication No. 03-5417. (2003).
Dickman, P. W. & Adami, H. O. Interpreting trends in cancer patient survival. J Intern Med 260, 103–117 (2006).
Hakulinen, T., Seppa, K. & Lambert, P. C. Choosing the relative survival method for cancer survival estimation. Eur J Cancer 47, 2202–2210 (2011).
Author information
Authors and Affiliations
Contributions
H.M. and X.S. planned the research. H.M. and H.S. calculated statistics and wrote the manuscript. X.S. supervised the entire project. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
SUPPLEMENTARY INFORMATION
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Ma, H., Sun, H. & Sun, X. Survival improvement by decade of patients aged 0–14 years with acute lymphoblastic leukemia: a SEER analysis. Sci Rep 4, 4227 (2014). https://doi.org/10.1038/srep04227
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep04227
This article is cited by
-
A novel prognostic nomogram for adult acute lymphoblastic leukemia: a comprehensive analysis of 321 patients
Annals of Hematology (2023)
-
Retrospective study of Dana Farber Consortium Protocol in newly diagnosed Egyptian adolescents and young adults with acute lymphoblastic leukemia: Tanta experience
Journal of the Egyptian National Cancer Institute (2021)
-
The impact of pretreatment serum cobalamin and folate levels on complications and peripheral blood recovery during induction chemotherapy of leukemia: a cross-sectional study
Supportive Care in Cancer (2021)
-
Cost-Effectiveness of Tisagenlecleucel in Paediatric Acute Lymphoblastic Leukaemia (pALL) and Adult Diffuse Large B-Cell Lymphoma (DLBCL) in Switzerland
Advances in Therapy (2021)
-
Antigen-independent activation enhances the efficacy of 4-1BB-costimulated CD22 CAR T cells
Nature Medicine (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.