Abstract
T-cell lymphomas are aggressive lymphomas with decreased prognosis and resistance to therapy. BAG-3 and heat shock protein 70 (HSP70) function in chemotherapeutic resistance and cellular survival. Expression of BAG-3 has not been investigated in T cell lymphomas. We investigated fifty cases including benign, systemic and cutaneous T cell lymphomas. Benign T cells were negative for BAG-3 and HSP70 immunohistochemical staining. BAG-3 expression correlated with increased HSP70 expression in a subset of systemic T cell lymphoma cases co-expressing the CD30 antigen. Correlation between BAG-3, HSP70 and CD30 expression was not seen in cutaneous T cell lymphoma cases. However, these cases showed a significant increase in BAG-3 staining when compared to CD30 negative systemic T cell lymphoma cases. The differential protein expression profile of BAG-3 and HSP70 may indicate a specific role for these proteins and the ubiquitin-proteasome system/autophagy in T cell lymphomas which may help guide future targeted therapy.
Similar content being viewed by others
Introduction
T-cell lymphomas account for 12% of all non-Hodgkin lymphomas and generally have a poor response to conventional chemotherapy and a low survival rate1. Consequently, targeted drug therapies are currently being investigated with the goal of improving therapeutic outcomes2. One type of targeted drug therapy includes inhibitors to the ubiquitin-proteasome system (UPS). The UPS functions in protein processing of bound “client” proteins by determining whether to protect and/or repair the client protein or target it for protein degradation3. This determination is dependent on the binding of specific proteins including heat shock protein 70 (HSP70) and heat shock protein 90 (HSP90). If the client protein is targeted for degradation through ubiquinization, it will be degraded in the proteasome.
Bortezomid, Lenalidomide and Geldenamycin are drugs which specifically target the UPS pathway. Bortezomid has been shown to specifically block the proteasome and thus inhibit client protein degradation4. Lenalidomide blocks upstream of the proteasome by acting on the protein cereblon which functions in the E3 ubiquitin ligase complex5,6. Geldenamycin and 17-AAG work on the chaperone protein HSP90, inhibiting its binding to the client protein7.
Due to the efficacy of Bortezomid and Lenalidomide in plasma cell myeloma patients, their utilization in B-cell lymphomas and T-cell lymphomas are under investigation in a number of clinical trials2,8,9,10. Although targeting the UPS through Bortezomid treatment results in sustained remission in plasma cell myeloma patients, resistance ultimately occurs. A number of resistance mechanisms have been proposed, one of which involves the autophagy pathway11. The autophagy pathway demonstrates degradation specificity similar to the UPS via HSP7012. One type of autophagy pathway known as the chaperone-assisted selective autophagy pathway also utilizes the protein BAG-3 in determining the removal and degradation of specific proteins13,14.
BAG-3 (CAIR-1, Bis) is an anti-apoptotic protein which functions as a co-chaperone protein in the UPS/autophagy pathway via its direct binding to HSP703,15. Reports have shown that overexpression of BAG-3 rescues cells from apoptosis under conditions of heat stress and chemotherapy treatment3,16.
Studies have evaluated the efficacy of UPS targeted therapy in T-cell lymphoma cell lines through inhibition of HSP90. Treatment of T-cell lymphoma cell lines with the HSP90 inhibitor 17-AAG resulted in increased cell death9. However, cellular resistance to HSP90 targeted inhibitors has been shown to occur through the action of the protein BAG-3 in melanoma cell lines3.
Overexpression of BAG-3 and HSP70 has also been shown to cause resistance to Bortezomid. Treatment of proximal renal tubular epithelial cells and glomerular mesangial cells with Bortezomid caused increased apoptosis and caspase activation in glomerular mesangial cells17. Microarray analysis showed an increased mRNA expression level of HSP70 and BAG-3 in proximal tubular epithelial cells compared with glomerular mesangial cells. This demonstrates that certain cell types may develop therapeutic resistance to Bortezomid via upregulation of BAG-3 and HSP70.
The aim of this study is to analyze the immunohistochemical expression profile of the anti-apoptotic protein BAG-3 and its protein partner HSP70 in benign and neoplastic T cells and their correlation with lymphoma subtype and immunophenotype.
Results
Clinical features
Fifty cases were analyzed and included eight reactive/benign lymphoid cases, 35 systemic T-cell lymphomas and seven primary cutaneous T-cell lymphomas (Tables 1, 2). The patients of systemic T-cell lymphoma cases had an age range between 2–88 years old (yo) with a median age of 52 yo and a male-to-female ratio of 1.2:1. Eighty-eight percent of the systemic T-cell lymphoma cases were high stage (stage III or IV) at presentation. Anaplastic large cell lymphoma ALK positive and negative and peripheral T-cell lymphoma, not otherwise specified composed a majority of the systemic T-cell lymphomas (13 cases and 15 cases, respectively). Additional subtypes included angioimmunoblastic T-cell lymphoma (two cases), T-cell prolymphocytic leukemia (two cases) and one case each of extranodal NK/T-cell lymphoma nasal type, hepatosplenic T-cell lymphoma and enteropathy-associated T-cell lymphoma. CD3 expression was evaluated in all cases and was positive in 71% of total cases and CD30 staining was positive in 62% (18 of 29 cases). The clinical findings and immunophenotype of each subtype is summarized in Table 1.
The median age for the primary cutaneous T-cell lymphoma cases was 66 yo with an age range from 44–69 yo (Table 2). Subtypes included primary cutaneous CD4 positive small/medium T-cell lymphoma (one case), cutaneous anaplastic large cell lymphoma (one case), mycosis fungoides (two case), large cell transformation of mycosis fungoides (one case), cutaneous involvement of PTCL (one case) and primary gamma-delta T cell lymphoma (one case). CD3 expression was evaluated in all cases and was positive in 86% of the cases and CD30 was positive in one case and showed partial positivity in a second case.
Evaluation of BAG-3 immunohistochemical expression in T-cell lymphomas
Evaluation of BAG-3 staining profile in T-cell lymphomas showed an increase in BAG-3 expression in systemic T-cell lymphomas co-expressing CD30 (Figure 1a–c). Sixteen of 18 cases of CD30 positive systemic T-cell lymphomas had a quantitative score of 2 and two cases had a quantitative score of 1 with an average quantitative score of 1.9 (Table 3). Systemic T-cell lymphomas with minimal or no expression of CD30 had an average BAG-3 quantitative score of 0.3; five of 17 cases had a quantitative score of 1 and the remainder of the cases had no staining for BAG-3 in the neoplastic cells (quantitative score of zero) (Figure 1e–g). The correlation between BAG-3 and CD30 expression in systemic T-cell lymphomas was statistically significant (p < 0.05).
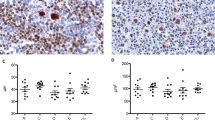
Immunohistochemical analysis of BAG-3 and HSP70 in T-cell lymphoma and benign cases (A).
H&E stain highlights large malignant T-cells in Anaplastic large cell lymphoma, ALK-1 negative case. (B). CD30 stain highlights diffuse strong CD30 positivity in neoplastic cells (C). BAG-3 stain highlights 2+ quantitative and qualitative staining pattern of BAG-3 in the neoplastic cells (D). HSP70 stain highlights 2+ quantitative and qualitative staining pattern of HSP70 in the neoplastic cells (E). H&E stain highlights malignant T-cells in a peripheral T- cell lymphoma, not otherwise specified case (F). CD30 stain highlights negative staining for CD30 in neoplastic cells (G). BAG-3 stain highlights 1+ staining pattern of BAG-3 in neoplastic cells (H). HSP70 stain highlights negative staining (0 pattern) in neoplastic cells (I). H&E stain highlights reactive lymphoid cells in adenoids (J). CD3 stain highlights numerous benign interfollicular T-cells (K). BAG-3, highlights negative (0 pattern) BAG-3 staining in the benign T-cells (L). HSP70 stain highlights negative (0 pattern) HSP70 staining in the benign T-cells (A-H 400×, I 100×, J-L 200×, see scale marker).
Based on diagnostic subtypes, BAG-3 overexpression was seen in all cases of systemic anaplastic large cell lymphoma with 11 of 13 cases showing a quantitative score of 2. The two remaining cases were extranodal locations of anaplastic large cell lymphoma (bone marrow and lung) and each had a quantitative score of 1. Thirty-three percent (five cases) of peripheral T-cell lymphoma, not otherwise specified, demonstrated a BAG-3 quantitative score of 2, all of which corresponding to increased CD30 expression. Twenty-seven percent (four cases) and 40% (six cases) of peripheral T-cell lymphoma, not otherwise specified contain a quantitative score of 1 and 0 respectively. One of the two cases of angioimmunoblastic T-cell lymphomas showed a BAG-3 quantitative score of 1. The remainder of the subtypes showed no increase in expression of BAG-3. The intensity of BAG-3 immunohistochemical staining was also evaluated; however there were no correlation with initial stage of disease, age at presentation or disease free interval.
All of the primary cutaneous T-cell lymphomas showed an overall increase in BAG-3 expression (average quantitative score of 1.6) which did not show correlation with the expression of CD30. Primary cutaneous T-cell lymphoma cases did show a significant increase in BAG-3 staining when compared to CD30 negative systemic T-cell lymphoma cases (p < 0.05) and showed a similar expression pattern when compared to CD30 positive systemic T-cell lymphoma cases.
Evaluation of inducible HSP70 immunohistochemical expression in T cell lymphomas
A subset of cases was further analyzed for expression of BAG-3's protein partner, HSP70. Twenty cases of systemic T-cell lymphoma, two cases of cutaneous T-cell lymphoma and six benign cases were evaluated for inducible HSP70 expression.
All CD30 positive systemic T-cell lymphomas showed an increase in HSP70 expression with an average quantitative score of 1.7 (Figure 1d). A similar staining pattern was seen in the expression of HSP70 and BAG-3 in CD30 positive systemic T-cell lymphomas. Only one case of CD30 negative systemic T-cell lymphomas demonstrated minimal expression of HSP70 (Figure 1h). The overall average quantitative score was 0.1. The correlation between HSP70 and CD30 expression in systemic T-cell lymphomas was statistically significant (p < 0.05). Two cutaneous T-cell lymphoma cases were analyzed. A primary cutaneous anaplastic large cell lymphoma case contained an HSP70 quantitative score of 2 while the cutaneous T-cell lymphoma showed no increase in HSP70 expression.
Evaluation of BAG-3 and inducible HSP 70 immunohistochemical expression in normal lymphoid tissues
IHC studies of BAG-3 on reactive/benign lymphoid tissue including tonsil/adenoids and spleen showed no immunoreactivity in the lymphoid cells, particularly the interfollicular areas rich in T-cells and periarteriolar lymphatic sheaths (Figure 1i–k). BAG-3 staining was seen in epithelial cells as well as endothelial cells with a cytoplasmic staining pattern, which was used as an internal control. Benign T-cells did not show an increase in expression of HSP70 (Figure 1l).
Discussion
The aim of the current study is to analyze the protein expression profile of the anti-apoptotic protein BAG-3 and protein binding partner HSP70 in benign and neoplastic T-cells. The results of our study show that benign T-cells show no increased expression of BAG-3 and inducible HSP70 protein via immunohistochemical analysis. This finding correlates with previous research demonstrating decreased BAG-3 mRNA in thymic and splenic tissue compared to other tissue types19. However, systemic T-cell lymphomas demonstrate a significant correlation between increased protein levels of BAG-3 and HSP70 in a subset of cases. Further analyses of the immunoprofile of known lymphoid antigens show that there is a statistically significant increase in the expression of BAG-3 and HSP70 in systemic T-cell lymphomas co-expressing the CD30 antigen. Thus BAG-3 and HSP70 were increased in anaplastic large cell lymphomas, regardless of ALK-1 co-expression and in a subset of peripheral T-cell lymphoma NOS correlating with increased CD30 co-expression. In cases with available clinical follow-up, the intensity of BAG-3 staining in CD30 positive systemic T-cell lymphomas is irrespective of initial stage of disease or disease free interval.
Interestingly, primary cutaneous T-cell lymphomas showed an overall increase in BAG-3 expression which did not correlate with CD30 co-expression. BAG-3 expression in primary cutaneous T-cell lymphomas was similar to CD30 positive systemic T-cell lymphomas and was significantly increased when compared with CD30 negative systemic T-cell lymphomas. These overall findings demonstrate a specific protein expression profile for BAG-3 and HSP70 in T-cell lymphomas.
BAG-3 is an anti-apoptotic protein which has been shown to play a pivotal role in cell survival and chemotherapeutic resistance. In epithelial cells, increased BAG-3 protein expression induced resistance to the HSP90 inhibitor geldenamycin3. BAG-3 expression was also shown to affect cell survival of a number of epithelial cancer cell lines when treated with the proteasome inhibitor MG132. Decreasing the levels of BAG-3 in cell lines transfected with BAG-3 small interfering RNA (siBAG-3) caused an increase in MG132 therapy induced apoptosis versus controls20. BAG- 3 expression also allows resistance to other commonly used chemotherapeutics such as Fludarabine21.
There are active investigations into the mechanism of BAG-3's anti-apoptotic effects. The interaction between BAG-3 and HSP70 was initially investigated in malignant melanoma cell lines3. The study by Doong et al. showed that increased BAG-3 levels through transfection studies showed a decrease in geldenamycin induced apoptosis. The findings showed that when BAG-3 bound with HSP70, there was a block in downstream client protein targeted degradation in the UPS. The anti-apoptotic protein AKT was one of the client proteins analyzed. Cells with increased BAG-3 levels contained AKT protein preservation and continued activation.
Another anti-apoptotic mechanism involves the NF-KB signaling pathway in osteosarcoma and melanoma cell lines22. The study by Ammirante et al. demonstrated that these two cell lines showed overexpression of BAG-3 and that upon inhibition of this overexpression, there was increased susceptibility to etoposide and serum deprivation induced apoptosis. The study found that through the interaction of HSP70 and BAG-3, IKK-gamma was protected from the UPS directed degradation allowing for continued activation of NF-KB and cellular survival. The NF-KB pathway is known to play an important role in cellular survival in Hodgkin and non-Hodgkin lymphomas and plasma cell myelomas23,24. In classical Hodgkin lymphoma and anaplastic large cell lymphoma, CD30 functions in the downstream activation of the NF-KB pathway25,26. Peripheral T cell lymphoma, NOS with CD30 expression has been shown to have decreased failure free and overall survival27. Our analysis demonstrates that in addition to NF-KB activation via CD30, these cells may also obtain continued NF-KB activation via BAG-3 and HSP70. Further research is thus warranted in the investigation of an augmentative role of BAG-3, possibly via continued activation of NF-KB, in CD30 positive T cell lymphomas.
Peripheral T-cell lymphomas incorporate a variety of histological and immunophenotypical subgroups1. Thus distinction of these subcategories based on overexpression of anti-apoptotic proteins may be needed for more specific targeted therapy since most T-cell lymphomas are known to have a poor response to conventional chemotherapy. Our findings show that the anti-apoptotic protein BAG-3 and HSP70 are selectively expressed in systemic T-cell lymphoma cases co-expressing CD30 and in cutaneous T-cell lymphomas regardless of CD30 expression. The current study shows a specific protein expression profile for BAG-3 and HSP70 in T-cell lymphomas. These findings indicate a potential function for these proteins in cellular survival in a subset of T-cell lymphomas compared with benign T-cells. However, some of the limitations of the study include evaluation on a small number of cases and somewhat weak HSP70 immunohistochemical staining. Thus, future investigation on a larger case cohort with analysis utilizing a more robust HSP70 stain is warranted in the evaluation of the role of BAG-3 and HSP70 in the UPS/autophagy pathway and T-cell lymphomagenesis.
Methods
Case selection
Fifty cases were reviewed from the Institutional Review Boards of Indiana University School of Medicine (EX0809-33) and Mayo Clinic, Jacksonville, FL (09-4711). Archival materials obtained from 2001 to 2009, including H&E stained slides, immunohistochemical staining and flow cytometric analysis were reviewed. Systemic and cutaneous T cell lymphoma cases with material available for additional analysis (paraffin block or unstained slides) were selected. Benign lymphoid cases with material available for additional analysis were selected for use as negative controls. Clinical information was extracted from medical records. All patient data were de-identified. The cases were reviewed independently (KAR, LJ, DMM) for evaluation of original diagnosis and staining characteristics. Cases in which there was disagreement as to the original diagnosis were not included in the study.
Immunohistochemical analysis and evaluation
Affinity purified rabbit Anti-BAG-3 (Novus Biologicals, Littleton, CO) antibody was utilized by immunohistochemical methods using a working dilution of 1:3000. The immunogen was a recombinant protein fragment corresponding to the C-terminal 196 amino acids of human BAG-3. Affinity purified rabbit Anti-HSP70 (Cell Signaling, Boston, MA) antibody was utilized by immunohistochemical methods using a working dilution of 1:50. The immunogen is a synthetic peptide (KLH-coupled) corresponding to human inducible HSP70. The antibody was validated utilizing breast carcinoma cells as positive controls and reactive lymphoid tissue as negative control. Earlier findings demonstrated positivity for HSP70 in centroblasts and immunoblasts and negativity in mantle cells and T-cells18. Initial immunophenotyping of all cases is by immunohistochemical and/or flow cytometric analysis. CD3 (IR503) immunohistochemical stain is a polyclonal rabbit anti-human antibody (DAKO, Carpinteria, CA) and CD30 (IR602) is a monoclonal mouse anti-human antibody (DAKO, Carpinteria, CA). CD30 immunohistochemical staining was diagnostically evaluated based on the number of positive tumor cells from quantitative recommendations from the 2008 WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues1. CD30 quantitation score approaching 100% was present in cases of anaplastic large cell lymphoma. Cases of peripheral T cell lymphoma with CD30 positivity ranged in CD30 quantitative expression from >10% to <75%.
Anti-BAG-3 and HSP70 immunohistochemical staining was evaluated on quantity of cells positive: 0 = negative, 1 = ≤25% positive, 2 = >25% positive; and quality of staining intensity: 0 = negative, 1 = weak/moderate, 2 = strong. Intensity was scored by the pathologist with strong staining intensity based on similarity to staining intensity to background positive controls. Disagreement between percent positivity were given a half point designation (1.5 versus 2), no disagreement as to negative versus positive staining was seen.
Tissue microarray analysis
A portion of the cases analyzed for HSP70 staining were constructed on a tissue microarray slide as follows: Recipient and donor blocks were prepared before tissue microarray assays (TMA) were constructed. Recipient block was prepared with regular paraffin in the laboratory and donor blocks for samples and controls were obtained from archived cases. The semi-automated TMArrayer™ was used to construct TMA blocks. TMA pattern was designed and core diameter (2.00 mm) was chosen after considering the number of evaluated samples and the site of the tumor. Targeted regions were marked by a pathologist. The construction of a TMA block included a hole-punch in a recipient block. Tissue was then taken from designated regions of the donor blocks then inserted into the recipient block.
Statistical analysis
The statistical associations of BAG-3 and HSP70 were analyzed by the Wilcoxon Two Sample Test where a p-value of < 0.05 was considered to be statistically significant. Averages and median values were analyzed via Microsoft excel spreadsheet.
References
Swerdlow, S. H. et al. WHO Classification Of Tumours of Haematopoietic And Lymphoid Tissues. 4th edn. WHO Publications Center, Albany (2008).
Zain, J. M. & O'Connor, O. Targeted treatment and new agents in peripheral T-cell lymphoma. Int J Hematol. 92, 33–44 (2010).
Doong, H. et al. CAIR-1/BAG-3 abrogates heat shock protein-70 chaperone complex-mediated protein degradation: accumulation of poly-ubiquitinated Hsp90 client proteins. J Biol Chem. 278, 28490–28500 (2003).
Mitchell, B. The Proteasome-An Emerging Therapeutic Target in Cancer. N Engl J Med. 348, 2597–2598 (2003).
Lopez-Girona, A., Mendy, D. & Ito, T. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia 26, 2326–2335 (2012).
Zhu, Y. X. et al. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood 118, 4771–4779 (2011).
Neckers, L. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends in Mol Med. 8, S55–S61 (2002).
Ruan, J. et al. Bortezomid Plus CHOP-Rituximab for Previously Untreated Diffuse Large B-Cell Lymphoma and Mantle Cell Lymphoma. J Clin Oncol. 29, 690–697 (2011).
Georgakis, G. V., Li, Y., Rassidakis, G. Z., Medeiros, L. J. & Younes, A. The HSP90 inhibitor 17-AAG synergizes with doxorubicin and U0126 in anaplastic large cell lymphoma irrespective of ALK expression. Exp Hematol. 34, 1670–1679 (2006).
Bonvini, P., Zorzi, E., Basso, G. & Rosolen, A. Bortezomib-mediated 26S proteasome inhibition causes cell-cycle arrest and induces apoptosis in CD-30+ anaplastic large cell lymphoma. Leukemia. 21, 838–842 (2007).
Wilkinson, S. & Ryan, K. Autophagy: an adaptable modifier of tumourigenesis. Curr Opin Genet Dev. 20, 57–64 (2010).
Kraft, C., Peter, M. & Hofmann, K. Selective autophagy: ubiquitin-mediated recognition and beyond. Nat Cell Bio. 12, 836–841 (2010).
Kaushik, S. & Cuervo, A. Chaperones in autophagy. Pharmacol Res. 66, 484–493 (2012).
Gamerdinger, M., Kaya, A., Wolfrum, U., Clement, A. M. & Behl, C. BAG3 mediates chaperone-based aggresome-targeting and selective autophagy of misfolded proteins. EMBO. 12, 149–156 (2011).
Rosati, A. et al. Apoptosis inhibition in cancer cells: a novel molecular pathway that involves BAG3 protein. Int J Biochem Cell Biol. 39, 1337–1342 (2007).
Rosati, A., Graziano, V., De Laurenzi, V., Pascale, M. & Turco, M. C. BAG3: a multifaceted protein that regulates major cell pathways. Cell Death Dis. 2, e141. 10.1038/cddis.2011.24 (2011).
Sarkozi, R. et al. Bortezomib-induced survival signals and genes in human proximal tubular cells. J Pharmacol Exp Ther. 327, 645–656 (2008).
Leopardi, O., Naughten, W., Giannulis, I., Mirra, M. & Frigo, B. HSP70 is selectively over-expressed in the blast cells of the germinal centres and paracortex in reactive lymph nodes. Histopathology. 39, 566–571 (2001).
Doong, J. et al. CAIR-1/BAG-3 forms an EGF-regulated ternary complex with phospholipase C-gamma and Hsp70/Hsc70. Oncogene. 19, 4385–95 (2000).
Wang, H. Q., Liu, H. M., Zhang, H. Y., Guan, Y. & Du, Z. X. Transcriptional upregulation of BAG3 upon proteasome inhibition. Biochem Biophys Res Commun. 365, 381–385 (2008).
Romano, M. F. et al. BAG3 protein controls B-chronic lymphocytic leukaemia cell apoptosis. Cell Death Differ. 10, 383–385 (2003).
Ammirante, M. et al. IKK{gamma} protein is a target of BAG3 regulatory activity in human tumor growth. Proc Natl Acad Sci. 107, 7497–7502 (2010).
Nishikori, M. Classical and Alternative NF-kappaB Activation Pathways and Their Roles In Lymphoid Malignancies. J Clin Exp Hematopathol. 45, 15–24 (2005).
Demchenko, Y. N. et al. Classical and/or alternative NF-kappaB pathway activation in multiple myeloma. Blood. 115, 3541–3552 (2010).
Chiarle, R., Voena, C., Ambrogio, C., Piva, R. & Inghirami, G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer. 8, 11–23 (2008).
Wright, C. W. & Duckett, C. S. The aryl hydrocarbon nuclear translocator alters CD30-mediated NF-kappaB-dependent transcription. Science. 323, 251–255 (2009).
Savage K, A. et al. ALK anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 111, 5496–5504 (2008).
Author information
Authors and Affiliations
Contributions
K.A.R. designed and initiated the study; K.A.R. and L.J. executed the study, including case material collection and review, clinical information retrieval, staining evaluation and scoring. K.A.R. wrote the main manuscript and L.J. edited the manuscript. Z.Z. participated in the case material collection and review, performed antibody validation, slide production and immunohistochemical staining. D.M.M. took part in staining evaluation and manuscript preparation. K.A.R. prepared all the images.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Jiang, L., Zhao, Z., Menke, D. et al. Correlation of BAG-3 and Heat Shock Protein 70 with CD30 expression in T-cell Lymphomas. Sci Rep 4, 3952 (2014). https://doi.org/10.1038/srep03952
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep03952
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.