Abstract
Recent theory suggests that global warming may be catastrophic for tropical ectotherms. Although most studies addressing temperature effects in ectotherms utilize constant temperatures, Jensen's inequality and thermal stress considerations predict that this approach will underestimate warming effects on species experiencing daily temperature fluctuations in nature. Here, we tested this prediction in a neotropical pseudoscorpion. Nymphs were reared in control and high-temperature treatments under a constant daily temperature regime and results compared to a companion fluctuating-temperature study. At constant temperature, pseudoscorpions outperformed their fluctuating-temperature counterparts. Individuals were larger, developed faster and males produced more sperm and females more embryos. The greatest impact of temperature regime involved short-term, adult exposure, with constant temperature mitigating high-temperature effects on reproductive traits. Our findings demonstrate the importance of realistic temperature regimes in climate warming studies and suggest that exploitation of microhabitats that dampen temperature oscillations may be critical in avoiding extinction as tropical climates warm.
Similar content being viewed by others
Introduction
Predicting how climate change will impact on biodiversity requires detailed understanding of the fitness consequences of temperature increase for tropical terrestrial arthropods, the most diverse animals on earth. With metabolic rate in ectotherms increasing exponentially with temperature1,2, recent theory suggests that the physiological effects of warming may be most severe for terrestrial ectotherms inhabiting tropical regions3,4. Although median surface temperatures in the tropics are projected to increase by 3.5–4.0°C by the end of the century5, tropical terrestrial ectotherms, such as arthropods and reptiles, are adapted to an already hot environment and therefore have high baseline temperatures and narrow thermal safety margins that may limit their capacity to tolerate even small increases in temperature3,4,6.
Most studies addressing the effects of temperature on fitness traits in ectotherms have been conducted under constant temperature conditions (reviewed in Refs. 7,8,9,10,11). However, this approach may yield unrealistic results for the many organisms that experience regular daily temperature fluctuations in nature. Although rarely taken into account in climate change research11,12, diurnal variance in temperature can potentially influence fitness traits and mathematical theory suggests that temperature fluctuations are likely to be particularly important for tropical ectotherms that function close to their critical maximum temperatures. Jensen's inequality is a mathematical property that applies broadly to biological processes, involving non-linear relationships, ranging from metabolism to species interactions13,14. It states that the average result of a nonlinear function,  , does not equal the function evaluated at the average value of x,
, does not equal the function evaluated at the average value of x,  , with variance in x lowering the response variable, if the function is decelerating and raising it, if the function is accelerating13. Because ectothermic metabolic rate is an accelerating function, i.e., it increases exponentially with temperature, the average metabolic rate of an ectotherm across a range of diurnal temperatures will always be greater than its metabolic rate at the average daily temperature14.
, with variance in x lowering the response variable, if the function is decelerating and raising it, if the function is accelerating13. Because ectothermic metabolic rate is an accelerating function, i.e., it increases exponentially with temperature, the average metabolic rate of an ectotherm across a range of diurnal temperatures will always be greater than its metabolic rate at the average daily temperature14.
While there is little seasonal variation in temperature in the tropics, temperatures typically vary by approximately 8°C on a regular daily basis (NOAA National Climate Data Center web site, http://www.ncdc.noaa.gov/). For example, at the El Claro site on Barro Colorado Island in lowland central Panamá, shaded air temperature ranged, on average, from a daily minimum of 23.3°C to a daily maximum of 31.5°C during the period 1994 to 200515. In a recent simulated climate warming study, carried out at fluctuating temperatures that closely mimicked this natural diurnal temperature cycle, the 3.5°C increase that has been predicted for the tropics by the end of the century5 had highly detrimental consequences for the neotropical pseudoscorpion, Cordylochernes scorpioides16. Although elevated temperature significantly reduced survival, size and level of sexual dimorphism, its greatest impact was on reproductive traits in the two sexes. At high temperature, males produced only 45% as many sperm as control males and females were rendered sterile.
Characteristics of its biology make C. scorpioides a model system for investigating the life history, morphological and reproductive consequences of climate warming in a tropical ectotherm. Sperm transfer in this pseudoscorpion is indirect, with males transferring discrete packets of sperm to females by means of a stalked spermatophore deposited on the substrate (Fig. 1a). Indirect sperm transfer provides a window on ejaculate characteristics that is absent in species that transfer sperm directly via copulatory organs. Immediately following spermatophore deposition, matings can be interrupted and sperm packets retrieved for evaluation of sperm quality and quantity17. Females are live bearing and nourish developing embryos in an external, translucent brood sac (Fig. 1b) that overlies the genital opening18,19. This ‘external womb’ mode of viviparous reproduction enables non-invasive monitoring of female reproductive status and embryological development20.
Male and female reproductive traits in Cordylochernes scorpioides.
(a) A sperm packet (stained red) with everted tube and evacuated sperm (stained green). (b) Ventral view of a female carrying a brood sac of approximately 100 early-stage embryos. The images are reproduced from Ref. 46.
The strongly negative effects of high temperature on fitness-related traits in C. scorpioides16 may have been at least partially the result of the fluctuating regime that subjected individuals to temperatures between 34°C and 35°C for several hours each day. Averaging daily temperature eliminates this upper temperature extreme, and, according to Jensen's inequality13, should reduce high temperature induced metabolic stress in this tropical ectotherm. Here, we tested this daily temperature regime hypothesis by repeating the simulated climate warming study, but substituted constant mean temperature for the diurnally fluctuating temperature regime. In a split-brood experiment, we randomly assigned newborn offspring from replicate full-sibling C. scorpioides families to control and high temperature treatments. The control and high temperatures were determined by averaging the diurnally fluctuating temperatures used in the previous study16. Nymphs were reared to adults and we assessed the effect of temperature treatment on survivorship, developmental rate and sexually monomorphic and dimorphic morphological traits, as well as on sperm number and viability and female reproductive function. In addition, to distinguish between long-term, developmental and short-term, adult-exposure temperature effects on male fertility and female reproductive function, we carried out a second experiment, in which adults were switched between temperature treatments 7 days before sperm assessment in males and mating in females. Data from the constant and fluctuating temperature studies were then pooled, in order to assess the effect of daily temperature regime on response to increased temperature in this pseudoscorpion.
For nearly all traits, including reproductive traits in both males and females, pseudoscorpions at constant temperature outperformed their fluctuating-temperature counterparts. Cordylochernes scorpioides individuals experiencing constant temperature developed faster and were significantly larger. Males produced more sperm and a higher percentage of viable sperm and females were more likely to become gravid and produced a greater number of early-stage embryos. The most striking impact of temperature regime involved short-term, adult exposure, with constant temperature alleviating high-temperature effects on reproductive traits. If these findings are found to be broadly applicable, the differing effects of constant versus fluctuating daily temperatures have important implications not only for the design and interpretation of simulated climate warming studies but also for the impact of warming on the community composition of tropical terrestrial ecosystems.
Results
Constant temperature regime
Life history and morphological traits
The high temperature (H) treatment, which involved a 3.5°C increase above the current mean temperature of 27.3°C (the control (C) treatment) in a forested region of central Panamá, had a significant impact on survivorship, developmental rate, morphology and level of sexual dimorphism. In the H treatment, the mean proportion of pseudoscorpions surviving from birth to adult was 0.67 compared to 0.80 in the control (F1,35 = 9.19, P = 0.0046). Temperature treatment did not significantly affect sex ratio (F1,35 = 1.62, P = 0.2113), but the average proportion of adult male offspring was lower in the H treatment (0.42) than in the control (0.47).
Developmental time at elevated temperature was significantly reduced in both sexes (main effect of temperature: F1,139 = 96.22, P < 0.0001; xx– ± SE: C females = 40.38 days ± 0.70, H females = 34.80 days ± 0.71; C males = 44.41 days ± 0.71, H males = 37.06 days ± 0.75). Females developed significantly more rapidly than males (main effect of sex: F1,139 = 18.19, P < 0.0001) but the interaction between sex and temperature was not significant (F1,139 = 1.03, P = 0.3113). More rapid maturation at high temperature was associated with significantly smaller size for all morphological traits in the two sexes (P < 0.0001). Reduced size and sex-specific effects of elevated temperature on morphology were most apparent in sexually dimorphic traits of the pedipalps, appendages that are used in prey capture and male combat21. Chela hand depth, the most sexually dimorphic trait (main effect of sex: F1,130 = 122.64, P < 0.0001), displayed a much larger reduction in males than in females (sex x treatment interaction: F1,130 = 21.58, P < 0.0001). Consequently, degree of sexual dimorphism, i.e., mean male size relative to mean female size, decreased from 127% in the control to 113% in the high temperature treatment (Fig. 2). Differences between the sexes in response to elevated temperature was less apparent in cephalothorax length, a sexually monomorphic trait, (main effect of sex: F1,139 = 0.85, P = 0.3588; sex x temperature interaction: F1,139 = 7.93, P = 0.0056). Within each sex, scaling between chela hand depth and cephalothorax length was unaffected by temperature (males: t202 = 1.22, P = 0.2237; females: t201 = 1.00, P = 0.3166). Across the two temperature treatments, however, the allometric coefficient (±SE) was significantly higher in males than in females (males: β = 1.430 ± 0.045; females: β = 0.841 ± 0.044; t404 = 9.367, P < 0.0001). These results indicate that differences between males and females in allometry can explain the reduced level of sexual dimorphism at high temperature.
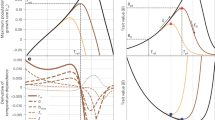
Size and level of sexual dimorphism decline significantly in response to high temperature.
Shown are means (±SE) of chela hand depth in males (triangles) and females (circles) under control and high temperature conditions. The image at the lower left indicates the measured trait, chela hand depth. Images of average-sized males from the control and high temperature treatments are shown at the upper left and lower right of the figure, respectively.
Male fertility traits
Temperature during development from birth to adult and temperature experienced as an adult were treated as fixed factors in these analyses, each with C and H treatment levels. We controlled for potential age and size effects on fertility traits in males by including male chela hand depth and male age as covariates, but neither significantly affected sperm number or sperm viability (P > 0.05). Continuous exposure to high temperature during development dramatically reduced sperm number (F1,79 = 56.00, P < 0.0001). Males that developed to sexual maturity at high temperature and remained at high temperature as adults (H males) produced only 32% as many sperm as control males (C males) (xx– ± SE: C males = 1,447 ± 112, H males = 457 ± 126). The short-term, 7-day exposure of C and H adult males to the alternative temperature treatment (C → H and H → C males) did not significantly affect sperm number (F1,79 = 0.26, P = 0.6137) but the developmental x adult treatment interaction effect was significant (F1,79 = 4.46, P = 0.0378). For sperm viability, the main effects of developmental temperature and adult temperature were not significant (F1,73 = 0.17, P = 0.6842 and F1,73 = 1.51, P = 0.2226, respectively). However, there was a significant interaction between developmental and adult temperature treatments, with a mean sperm viability (±SE) of 0.91 ± 0.02 in H males compared to 0.96 ± 0.02 in C males (F1,73 = 5.21, P = 0.0253).
Female reproduction
Fecundity was evaluated by counting early-stage embryos and female reproductive success was quantified based on the number of nymphs born to each female (see Ref. 19 for details). Female reproductive success is a function of both the female's fecundity and the fertility of her mate. We therefore assessed the effect of temperature experienced throughout development and adulthood on reproduction in females, by pairing virgin females and males in all four possible treatment combinations: C♀xC♂ (n = 16); C♀xH♂ (n = 16); H♀xC♂ (n = 15) and H♀xH♂ (n = 13). The proportion of females that produced a brood sac containing early-stage embryos varied significantly across the four female x male mating combinations (Fisher's exact test P = 0.0105), with only five of 28 H females (H♀xC♂ and H♀xH♂) becoming gravid (Fig. 3). Females reared and maintained as adults at high temperature produced significantly fewer early-stage embryos than their control temperature counterparts (xx– ± SE: 3.29 ± 4.32 versus 27.91 ± 3.97; main effect of female temperature treatment, F1,55 = 16.39, P = 0.0002). In addition, females mated to males reared at high temperature produced significantly fewer embryos than females mated to control males (xx– ± SE: 1.06 ± 4.19 versus 30.13 ± 4.11; main effect of male temperature treatment, F1,55 = 24.46, P < 0.0001). Male treatment effects were limited to C females because very few H females produced embryos. There was nonetheless a significant interaction between male and female treatment effects (F1,55 = 12.28, P = 0.0009; Fig. 4). The pattern for number of nymphs born was similar to that for early embryo production (main effect of female treatment: F1,55 = 17.41, P = 0.0001; main effect of male treatment: F1,55 = 19.95, P < 0.0001; female treatment x male treatment: F1,55 = 17.41, P = 0.0001).
Temperature treatment experienced by females and their mates significantly affects embryo production.
The mean (±SE) number of early-stage embryos is shown for the four experimental treatments. C and H (control and high temperature, respectively) indicate temperature treatment experienced by an individual throughout development and adulthood.
Switching females between high and control incubators 1 week prior to mating demonstrated a rescuing effect of short-term adult exposure to the control temperature for females that had developed at high temperature, with 18 of 29 producing embryos, compared to three of 15 H females maintained at high temperature as adults (Fisher Exact Test, P = 0.0114). By contrast, 18 of the 30 C females switched to the H environment became gravid, compared to 11 of 16 C females maintained as adults at the control temperature, indicating that females may be resistant to short-term exposure to high temperature as adults (Fisher Exact Test, P = 0.7500). For numbers of embryos produced and nymphs born, developmental and adult temperature regimes were again analyzed as fixed factors, each with C and H treatment levels. Both the temperature at which females developed (F1,67 = 10.14, P = 0.0022) and the short-term temperature exposure at the adult stage (F1,67 = 8.04, P = 0.0060) had statistically significant effects on the average number of early-stage embryos produced. The interaction between developmental and adult treatments was not significant (F1,67 = 0.46, P = 0.4979). This pattern was also evident for the number of nymphs born (main effect of developmental treatment: F1,67 = 14.76, P = 0.0003; main effect of adult treatment: F1,67 = 13.08, P = 0.0006; developmental treatment x adult treatment: F1,67 = 1.76, P = 0.1888).
Comparison of constant versus diurnally fluctuating temperature regime effects
For these analyses, data from this constant diurnal temperature study and the previous diurnally-fluctuating temperature investigation16 were pooled and temperature regime incorporated as an additional fixed factor in the models. Temperature regime did not significantly affect the impact of the projected 3.5°C increase in temperature on survival from birth to the adult stage or the proportion of male offspring produced (P > 0.05; data not shown). However, there were significant regime effects on developmental time, morphological traits, male fertility traits and female reproductive function.
The effect of regime on development time was large and highly significant (F1,265 = 66.75, P < 0.0001), with exposure to constant temperatures reducing developmental period for all possible regime x temperature x sex treatment comparisons (Fig. 5). For both the sexually dimorphic trait, chela hand depth and the sexually monomorphic trait, cephalothorax length, there was a significant main effect of regime, with larger size under the constant temperature regime (chela hand depth: F1,256 = 6.06, P = 0.0145; cephalothorax length: F1,265 = 4.41, P = 0.0366) and a significant interaction between regime and temperature (chela hand depth: F1,256 = 12.71, P = 0.0004; cephalothorax length: F1,265 = 6.00, P = 0.0150).
Sperm number was also significantly higher under the constant temperature regime (F1,173 = 5.96, P = 0.0156). Exposure to fluctuating temperatures decreased sperm number for all regime x temperature combinations, with the effect of regime most apparent in C males switched to high temperature as adults (Fig. 6). A similar pattern was evident for sperm viability (F1,154 = 11.97, P = 0.0007; Fig. 7). Overall, females were much more likely to become gravid under the constant temperature regime than under the fluctuating regime (56% versus 25%) and this difference was highly significant (Fisher Exact Test, P = 0.0001). While the proportion of females becoming gravid was higher for all female treatment categories under constant temperature, the effect of regime was again most apparent in the control to high temperature switch treatment. Sixty percent of the 30 constant regime C → H females became gravid, compared to 0% of the 21 fluctuating regime C → H females (Fisher Exact Test, P < 0.0001). The effect of regime on number of early stage embryos produced by females was also statistically significant (F1,122 = 9.66, P = 0.0023). As with sperm number and sperm viability, exposure to constant temperatures resulted in a higher number of embryos for all possible regime by temperature treatment combinations, but again, the effect of regime was greatest in C→H treatment (Fig. 8). Although females also gave birth to more nymphs at constant temperature than under fluctuating temperature conditions, the main effect of regime on number of protoymphs born was not statistically significant (F1,122 = 1.91, P = 0.1695).
Discussion
In a recently published study carried out under diurnally fluctuating temperature regimes designed to simulate current and predicted temperatures in the species' natural environment, the projected 3.5°C increase in tropical temperatures by the end of the 21st century had extremely detrimental consequences for important fitness-related traits in the harlequin beetle riding pseudoscorpion, C. scorpioides16. In the companion study reported here, the same experimental design performed under constant diurnal temperatures yielded results that were qualitatively but not quantitatively similar to the fluctuating temperature regime study. In both studies, the projected increase in temperature resulted in a marked reduction in survival, size, level of sexual dimorphism and sperm number and dramatically decreased female fecundity and reproductive success. However, for most of the assayed fitness-related traits, pseudoscorpions exposed to constant temperatures were superior to their fluctuating-temperature counterparts. At constant temperature, C. scorpioides individuals were larger and developed faster than pseudoscorpions that experienced diurnally fluctuating temperatures. Males produced more sperm and a higher percentage of viable sperm and females were more likely to become gravid and produced a greater number of early-stage embryos.
The strong effects of daily temperature regime reported here underscore the importance of taking into account both Jensen's inequality13 and thermal stress-mediated physiological disruption in investigating the effects of climate warming on tropical ectotherms. Because ectothermic metabolic rate increases exponentially with temperature, the average metabolic rate of an ectotherm across a range of diurnal temperatures should exceed its metabolic rate at the average daily temperature. This variance-driven increase in metabolic rate is likely to reduce both the optimum and critical maximum temperatures, as has recently been demonstrated empirically in the mosquito, Anopheles stephensi and theoretically in four tropical insect species11. In addition, under fluctuating high temperature regimes, daily exposure to temperatures approaching the critical maximum temperature for tropical arthropods is likely to generate physiological stress, damage associated with the metabolic production of reactive oxygen species22, and/or stress-induced activation or increased expression of transposable elements23,24,25. Heat shock experienced during larval development has been shown to disrupt adult wing morphology in Drosophila26 and genes regulating physiological responses to heat shock appear to be particularly vulnerable to transposable element-induced mutagenesis resulting from the insertion of P elements into their promoters27. With diurnal temperatures in the tropics typically varying by approximately 8°C (NOAA National Climate Data Center web site, http://www.ncdc.noaa.gov/), studies based on constant temperature regimes are likely to significantly underestimate the negative effects of climate warming on the many tropical terrestrial ectotherms that experience daily fluctuations in temperature.
In our study, depending on the traits involved, both variance-driven and stress-related mechanisms appear responsible for the reduced performance of individuals subjected to fluctuating temperatures. Temperature regime did not significantly affect survival and sex-specific survival to the adult stage, but fluctuating temperatures did result in small but significant reductions in developmental rate and body size, which, taken together, are consistent with predictions of Jensen's equality. However, the effects of temperature regime were most marked in the experimental treatments involving short-term, adult exposure, with constant temperature alleviating high-temperature effects on reproductive traits. For example, at constant temperature, the sperm packets of males switched to the elevated temperature as adults contained an average of 1,646 sperm, compared to an average of only 1,051 for C → H males under the fluctuating temperature regime (Fig. 6). Similarly, at constant temperature, females switched as adults to high temperature produced an average of 35.73 embryos, while none of their counterparts produced embryos under fluctuating temperature conditions (Fig. 8). These results suggest that physiological stress resulting from an increase in daily maximum temperature rather than increase in mean daily temperature is the most critical factor undermining reproductive success in this pseudoscorpion and that C. scorpioides could at least partially circumvent the threat posed by climate warming through adult exploitation of microhabitats that dampen daily fluctuations in temperature. At a broader level, species that reproduce and develop in exposed environments, such as phytophagous beetles, bugs and butterflies, might be at much greater risk from climate warming than species, such as ants, bees and termites with subterranean nests, whose microhabitat use and/or social systems buffer diurnal temperature fluctuations.
It is important to acknowledge that several factors, in addition to microhabitat-based dampening of diurnal temperature fluctuations could alleviate the negative impacts of climate warming on tropical ectotherms. From an abiotic perspective, climate models predict that diurnal temperature range will decrease due to greenhouse warming, with daily maximum temperatures rising less rapidly than daily minimum temperatures5. However, this prediction has received equivocal empirical support, with diurnal range declining through the mid 1980s28 but then followed by an increase between 1987 and 201029. Tropical ectotherms could respond to elevated temperature by range shifts to higher elevations or higher latitudes, although latitudinal range shifts in the tropics are complicated by shallow latitudinal temperature gradients30. However, shifting range boundaries upslope is a plausible option for C. scorpioides and other saproxylic species in mountainous regions of Central and South America. In the province of Chiriqui in western Panamá, for example, the elevational range of this pseudoscorpion spans at least 1500 m31. Nonetheless, warming-driven upward shifts in range are likely to result in the loss of considerable biodiversity, since tropical lowlands have no source pool of species that have adapted to withstand higher temperatures30. Finally, populations of tropical ectotherms could respond through acclimation32, reduced metabolic sensitivity33 or adaptation34 to elevated temperatures, although recent studies suggest that tropical ectotherms have limited heritable genetic variation for heat resistance35,36.
The natural history of C. scorpioides illustrates the complexity of species interactions in tropical ecosystems and their vulnerability to climate warming. Cordylochernes scorpioides is distributed throughout rain forests in Central and South America and inhabits decaying trees in the families Moraceae and Apocynaceae, particularly Ficus species. The pseudoscorpion disperses between these rich but patchily-distributed and ephemeral habitats by hitchhiking under the elytra of the giant harlequin beetle, Acrocinus longimanus21. Dying and newly dead trees bring in A. longimanus adults for mating, oviposition and larval development. The wood-boring activity of the beetle larvae then creates a microhabitat of exfoliating bark and frass that is exploited by a diverse community of invertebrate species that colonize the decaying tree31,37. While the significance of fig trees as keystone resources for folivorous and frugivorous vertebrates and invertebrates has been extensively documented (reviewed in Ref. 38), the post-mortem importance of Ficus in providing a habitat for saproxylic invertebrates that play an essential role in nutrient recycling is less well appreciated.
Ficus species are unique in possessing an enclosed inflorescence, the syconium and are pollinated through reciprocally obligate relationships with agaonid fig wasps that have an adult life span of only 1–2 days39. Although evaporative cooling in developing syconia moderates temperatures that would otherwise be lethal to fig wasp larvae40, recent research suggests that an increase of 3°C in daytime temperature would reduce adult lifespan in four species of fig wasps by 33%–60%41. If fig wasps are more vulnerable to increased temperature than longer-lived tropical species, the fate of the harlequin beetle riding pseudoscorpion and the myriad of other invertebrates and vertebrates that depend on fig trees may ultimately hinge on the ability of fig wasps to live long enough to disperse and pollinate Ficus in a rapidly warming tropical climate.
Methods
With the exception of constant temperature, the experimental methods employed in this study were identical to those reported for the diurnally fluctuating temperature study16 and will therefore be described only briefly here.
Study population and design of experiment
Cordylochernes scorpioides were drawn from a subsequent generation of the same large laboratory population used in the diurnally fluctuating temperature regime investigation16. To provide full-sibling families for this study, one virgin female from each of 36 families was mated to a randomly selected, unrelated male. Thirty of these families were derived from the same matrilines represented in the diurnally fluctuating temperature study16. We employed a split-brood design and randomly assigned 24 first-stage nymphs (protonymphs) from each female, 12 to a 27.3°C control treatment (C) and 12 to a 30.8°C high temperature treatment (H). These temperatures were determined by averaging the diurnally fluctuating temperatures used in the previous study16. Identical Percival I-36NL incubators were used for the H and C temperature treatments, the incubators were located in the same room and relative humidity was maintained at 85% in both temperature treatments. Nymphs were reared in individual vials to ensure virginity, as described elsewhere42. Developmental time was determined by daily visual inspection of vials for the presence of an adult during the period between 27 days and 70 days after birth. We emptied and inspected vials not yielding an adult after 70 days. If no nymphs or adults were found, individuals in these vials were scored as having died during development. The two sexes are morphologically indistinguishable as nymphs and gender was therefore assessed only for individuals that survived to the adult stage (n = 620). The effect of high temperature on male and female survivorship was assessed, using a paired comparison by full-sibling family, of the proportion of survivors that were male in the two temperature treatments.
Morphometrics
To evaluate high temperature effects on male and female size and degree of sexual dimorphism, adult morphometric data were obtained by holding individuals flat under a glass slide with the right pedipalp fully extended and photographing them at high magnification (approximately 30×)16. ImageJ 1.43 (National Institutes of Health, USA) was then used to measure five pedipalp and cephalothorax trait for a large sample of these images (n = 488). These traits, i.e., movable finger length, chela hand length, chela hand depth, tibia depth and cephalothorax length, are fixed in size at the final molt to the adult stage. We used principal components analysis to extract composite measures of size (PC1) and shape (PC2) from these five traits. As in the previous study16, hand depth in males (r = 0.99) and cephalothorax length in females (r = 0.92) were found to be the traits most closely correlated with PC1 score. Because of the strength of these correlations, female cephalothorax length and male hand depth were used as size covariates in analyses of treatment effects on female and male reproductive function (see below).
Male fertility traits
We evaluated male fertility traits for a subset of males (n = 139), reared and maintained at either the high or the control temperature, by staging matings between experimental males and non-experimental, virgin females, collecting sperm packets and quantifying sperm number and sperm viability, as described elsewhere17. Briefly, each male was placed in an arena with a female and the mating was observed under a stereomicroscope. Immediately after the spermatophore was deposited, the mating was interrupted and the sperm packet collected. Sperm packets were ruptured in phosphate buffered saline and stained with SYBR 14 and propidium iodide (Invitrogen Live/Dead Sperm Viability Kit). Each 11 μL stained sample was pipetted onto a hemocytometer and viewed under an Olympus BX51 fluorescence microscope. We estimated total number of sperm by multiplying sperm counted in a 0.9-μL volume of the sample by a factor of 12.2 (11 μL/0.9 μL). Sperm viability was estimated as the proportion of live sperm in the sample. In addition, 20 C males and 15 H males were switched between treatment incubators seven days before mating, in order to distinguish between long-term, developmental and short-term, adult exposure effects of temperature on male reproductive traits. Sperm number and sperm viability were then measured for these switch treatment males, as indicated above.
Female reproductive function
Virgin females were paired with males in the four possible treatment combinations (C♀xC♂; C♀xH♂; H♀xC♂ and H♀xH♂). Pairs were placed in a mating arena and given 40 min to mate. Typically, males produce a spermatophore within 7 min of encountering a female and previous research has established that 98% of virgin females are sexually receptive20,43. Cordylochernes scorpioides mated females exhibit one of the three possible outcomes: (i) failure to become gravid; (ii) production of a brood sac containing embryos followed by spontaneous abortion of the entire brood, or (iii) brood production, in which embryos are carried to term and nymphs are born simultaneously. Beginning on the fifth day after mating, each female was monitored until she gave birth, spontaneously aborted her brood of embryos or failed to become gravid within 30 d. Females that became gravid were gently removed from their vials as soon as individual embryos became clearly discernable and digital images were taken of their brood sacs for early-stage embryo counting, as described elsewhere19. We then returned females their vials and monitored them until they gave birth or spontaneously aborted the brood. Females remain in a silken nest constructed on the vial wall throughout gestation and embryonic development and brood status can therefore be monitored without further disturbance to the female20. Nymphs were removed from the nest and counted within 24 h of birth.
In order to differentiate between long-term, developmental and short-term, adult-exposure temperature effects on the ability of females to reproduce, we carried out a second experiment, in which, seven days prior to mating, 30 females from the control treatment and 29 females from the high temperature treatment were switched between temperature treatment incubators. We controlled for male effects in this “switch” experiment by mating all females to C males. Matings were carried out and female fecundity and reproductive success were evaluated, as described above.
Statistical analyses
Temperature effects on survival, developmental time, morphology and male and female reproductive traits under the constant diurnal temperature regime were analyzed, using a general linear mixed model (GLMM), as performed in PROC GLIMMIX in SAS, v9.344. We avoided pseudoreplication and controlled for genetic and maternal effects by including full-sibling family identity in the models as a random effect. Because morphological traits were normally or approximately normally distributed, GLMMs for analyzing these variables incorporated a Gaussian (Normal) distribution, an identity link function, a Laplace maximum likelihood approximation and the SAS containment method for determining degrees of freedom44,45. Embryo and protonymph count data were square root transformed and analyzed as above. To accommodate non-normality and overdispersion in the developmental time and sperm number data, GLMMs for analyzing these variables incorporated a log link function, a Gauss-Hermite Quadrature maximum likelihood approximation and a generalized Poisson mixed model for overdispersed count data (see Ref. 44, pp. 3123–3124). Sperm viability was evaluated as the proportion of live sperm in an ejaculate and was not normally distributed. We therefore analyzed this response variable, using the GLIMMIX logit link function to fit a binomial response variable44.
To assess the effects of experimental diurnal temperature regime on C. scorpioides' response to the 3.5°C projected temperature increase in the tropics, data from this constant daily temperature study and the previous fluctuating temperature study16 were pooled and analyzed, as above, with diurnal temperature regime incorporated as an additional fixed factor in the models. For all hypothesis testing, we used two-tailed P values.
References
Gillooly, J. F., Brown, J. H., West, G. B., Savage, V. M. & Charnov, E. L. Effects of size and temperature on metabolic rate. Science 293, 2248–2251 (2001).
Clark, A. Temperature and the metabolic theory of ecology. Funct. Ecol. 20, 405–412 (2006).
Deutsch, C. A. et al. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. USA 105, 6668–6672 (2008).
Dillon, M. E., Wang, G. & Huey, R. B. Global metabolic impacts of recent climate warming. Nature 467, 704–706 (2010).
IPCC. Climate Change 2007. In: The Physical Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (eds Solomon, S. et al.) Cambridge University Press, Cambridge, UK (2007).
Addo-Bediako, A., Chown, S. L. & Gaston, K. J. Thermal tolerance, climatic variability and latitude. Proc. R. Soc. Lond. B 267, 739–745 (2000).
Terblanche, J. S., Nyamukondiwa, C. & Kleynhans, E. Thermal variability alters climatic stress resistance and plastic responses in a globally invasive pest, the Mediterranean fruit fly (Ceratitis capitata). Entomol. Exp. Appl. 137, 304–315 (2010).
Estay, S. A., Clavijo-Baquet, S. M. & Bozinovic, F. Beyond average: an experimental test of temperature variability on the population dynamics of Tribolium confusum. Popul. Ecol. 53, 53–58 (2011).
Fischer, K., Kölzow, N., Höltje, H. & Karl, I. Assay conditions in laboratory experiments: is the use of constant rather than fluctuating temperatures justified when investigating temperature-induced plasticity? Oecologia 166, 23–33 (2011).
Chen, C.-Y., Chiu, M.-C. & Kuo, M.-H. Effect of warming with temperature oscillations on a low-latitude aphid, Aphis craccivora. Bull. Entomol. Res. 103, 406–413 (2013).
Paaijmans, K. P. et al. Temperature variation makes ectotherms more sensitive to climate change. Glob. Change Biol. 19, 2373–2380 (2013).
Blanford, J. I. et al. Implications of temperature variation for malaria parasite development across Africa. Sci. Rep. 3, 1300 (2013).
Jensen, J. L. Sur les fonctions convexes et les inégalités entre les valeurs moyennes. Acta Math. 30, 175–193 (1906).
Ruel, J. J. & Ayres, M. P. Jensen's inequality predicts effects of environmental variation. Trends Ecol Evol 14, 361–366 (1999).
Paton, S. Meteorological and hydrological summary for Barro Colorado Island. Smithsonian Tropical Research Institute (1994–2005
Zeh, J. A. et al. Degrees of disruption: projected temperature increase has catastrophic consequences for reproduction in a tropical ectotherm. Glob. Change Biol. 18, 1833–1842 (2012).
Bonilla, M. M., Zeh, D. W., White, A. M. & Zeh, J. A. Discriminating males and unpredictable females: males bias sperm allocation in favor of virgin females. Ethology 117, 740–748 (2011).
Weygoldt, P. The Biology of Pseudoscorpions. Harvard University Press, Cambridge (1969).
Koop, J., Zeh, D. W., Bonilla, M. M. & Zeh, J. A. Reproductive compensation favours male-killing Wolbachia in a live-bearing host. Proc. R. Soc. Lond. B 276, 4021–4028 (2009).
Newcomer, S. D., Zeh, J. A. & Zeh, D. W. Genetic benefits enhance the reproductive success of polyandrous females. Proc. Natl. Acad. Sci. USA 96, 10236–10241 (1999).
Zeh, D. W., Zeh, J. A. & Bermingham, E. Polyandrous, sperm-storing females: carriers of male genotypes through episodes of adverse selection. Proc. R. Soc. Lond. B 264, 119–125 (1997).
Yang, L. H., Huang, H. & Wang, J. J. Antioxidant responses of citrus red mite, Panonychus citri (McGregor) (Acari: Tetranychidae), exposed to thermal stress. J. Insect Physiol. 56, 1871–1876 (2010).
Ratner, V. A., Zabanov, S. A., Kolesnikova, O. V. & Vasilyeva, L. A. Induction of the mobile genetic element Dm-412 transpositions in the Drosophila genome by heat-shock treatment. Proc. Natl. Acad. Sci. USA 89, 5650–5654 (1992).
de la Vega, E. et al. Stress-induced gene expression profiling in the black tiger shrimp Penaeus monodon. Physiol. Genomics 31, 126–138 (2007).
Desalvo, M. K. et al. Differential gene expression during thermal stress and bleaching in the Caribbean coral Montastraea faveolata. Mol. Ecol. 17, 3952–3971 (2008).
Williams, K. D., Helin, A. B., Posluszny, J., Roberts, S. P. & Feder, M. E. Effect of heat shock, pretreatment and hsp70 copy number on wing development in Drosophila melanogaster. Mol. Ecol. 12, 1165–1177 (2003).
Walker, J.-C., Chen, B. & Feder, M. E. Heat-shock promoters: targets for evolution by P transposable elements in Drosophila. PLoS Genet 2, e165 (2006).
Vose, R. S., Easterling, D. R. & Gleason, B. Maximum and minimum temperature trends for the globe: An update through 2004. Geophys. Res. Lett. 32, L23822 (2005).
Rohde, R. et al. A new estimate of the average earth surface land temperature spanning 1753 to 2011. Geoinfor Geostat: An overview. 1, 1 (2012).
Colwell, R. K., Brehm, G., Cardelús, C. L., Gilman, A. C. & Longino, J. T. Global warming, elevational range shifts and lowland biotic attrition in the wet tropics. Science 322, 258–261 (2008).
Zeh, J. A., Zeh, D. W. & Bonilla, M. M. Phylogeography of the harlequin beetle-riding pseudoscorpion and the rise of the Isthmus of Panamá. Mol. Ecol. 12, 2759–2769 (2003).
Mitchell, K. A. & Hoffmann, A. A. Thermal ramping rate influences evolutionary potential and species differences for upper thermal limits in Drosophila. Funct. Ecol. 24, 694–700 (2010).
Williams, C. M. et al. Thermal variability increases the impact of autumnal warming and drives metabolic depression in an overwintering butterfly. PLoS ONE 7, e34470 (2012).
Williams, B. R., Van Heerwaarden, B., Dowling, D. K. & Sgrò, C. M. A multivariate test of evolutionary constraints for thermal tolerance in Drosophila melanogaster. J. Evol. Biol. 25, 1415–1426 (2012).
Overgaard, J., Kristensen, T. N., Mitchell, K. A. & Hoffman, A. A. Thermal tolerance in widespread and tropical Drosophila species: does phenotypic plasticity increase with latitude? Am. Nat. 178, S80–S96 (2011).
Grigg, J. W. & Buckley, L. B. Conservatism of lizard thermal tolerances and body temperatures across evolutionary history and geography. Biol. Lett. 9, 20121056 (2013).
Zeh, J. A. & Zeh, D. W. Tropical liaisons on a beetle's back. Nat. Hist. 103, 36–43 (1994).
Herre, E. A., Jandér, K. C. & Machado, C. A. Evolutionary ecology of figs and their associates: recent progress and outstanding puzzles. Annu. Rev. Ecol. Evol. Syst. 39, 439–458 (2008).
Cook, J. M. & Rasplus, J.-Y. 2003 Mutualists with attitude: coevolving fig wasps and figs. Trends Ecol. Evol. 18, 241–248 (2003).
Patino, S., Herre, E. A. & Tyree, M. T. Physiological determinants of Ficus fruit temperature and implications for survival of pollinator wasp species: comparative physiology through an energy budget approach. Oecologia 100, 13–20 (1994).
Jevanandam, N., Goh, A. G. R. & Corlett, R. T. Climate warming and the potential extinction of fig wasps, the obligate pollinators of figs. Biol. Lett. 9, 20130041 (2013).
Zeh, D. W., Zeh, J. A. & Bonilla, M. M. Wolbachia, sex ratio bias and apparent male killing in the harlequin beetle riding pseudoscorpion. Heredity 95, 41–49 (2005).
Zeh, J. A., Newcomer, S. D. & Zeh, D. W. Polyandrous females discriminate against previous mates. Proc. Natl. Acad. Sci. USA 95, 13732–13736 (1998).
SAS Institute Inc. SAS/STAT® 9.3 User's Guide. Cary, NC: SAS Institute Inc. (2011).
Ferron, J. M., Bell, B. A., Hess, M. R., Rendina-Gobioff, G. & Hibbard, S. T. Making treatment effect inferences from multiple-baseline data: the utility of multilevel modeling approaches. Behav. Res. Meth. 41, 372–384 (2009).
Zeh, J. A., Bonilla, M. M., Adrian, A. J., Mesfin, S. & Zeh, D. W. From father to son: transgenerational effect of tetracycline on sperm viability. Sci. Rep. 2, 375 (2012).
Acknowledgements
We thank La Autoridad Nacional del Ambiente (A.N.A.M.) for permission to collect pseudoscorpions in Panamá, the Smithsonian Tropical Research Institute for extensive logistical support. This research was supported by grants from the National Geographic Society and the National Science Foundation (DEB-0721226) to J.A.Z. and D.W.Z.
Author information
Authors and Affiliations
Contributions
J.A.Z. designed the study, J.A.Z., M.M.B., E.J.S., M.V.P. and R.V.A. performed the experiments, D.W.Z. analyzed the data and J.A.Z. and D.W.Z. wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Zeh, J., Bonilla, M., Su, E. et al. Constant diurnal temperature regime alters the impact of simulated climate warming on a tropical pseudoscorpion. Sci Rep 4, 3706 (2014). https://doi.org/10.1038/srep03706
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep03706
This article is cited by
-
Experimental heatwaves compromise sperm function and cause transgenerational damage in a model insect
Nature Communications (2018)
-
Large diurnal temperature range increases bird sensitivity to climate change
Scientific Reports (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.