Abstract
The finding that mature forest ecosystems increase carbon in woody tissues and mineral soils indicates that the original equilibriums are being pushed to a higher state. The final driving forces will probably be increasing CO2 and nitrogen deposition, global warming and changes to precipitation patterns. However, which part of a mature forest bears the direct impacts of environmental changes and reactivates the balanced ecosystem processes remains unclear. Here, we investigated the living biomass of mature forests in the tropical and subtropical biomes in China and found that active organs and small individuals have accumulated carbon at a rate of 203 kg C ha−1 yr−1 in recent decades, whereas the woody tissues did not display carbon accumulation with statistical significance. Our findings indicate that the increased labile plant inputs may have shifted mature forests from their previous equilibrium and caused them to enter a new non-equilibrium state.
Similar content being viewed by others
Introduction
Mature forests are considered to be carbon-neutral as well as to be in balanced states for all other ecosystem processes1, including the process that links living biomass to soil organic carbon. Previous studies have demonstrated that the carbon in the biomass of boreal, temperate and tropical old-growth forests and the soil organic carbon in subtropical old-growth forests2 have increased over recent decades3,4,5,6. In these previous studies, the biomass carbon refers only to the “inactive carbon” that is stored in the woody tissues of large trees5,6,7; it does not include the biomass carbon in active organs (leaves, roots) and small individuals (small arbor and shrub, or small diameter at breast height (DBH) trees) that can also be considered “active carbon”.
Differences in some ecosystem functions between active and inactive biomass carbon are apparent. Table 1 presents some characteristics of the two classifications that relate to this study.
Although active carbon does not appear to have a role in mitigating the rising atmospheric CO2 due to its short residence time, it plays an irreplaceable role in ecosystem processes8,9 involving the formation of ecosystem net productivity (NEP)10,11. Increased active carbon in a mature forest may imply a reactivation of the ecosystem functions that can drive the mature forest into a new equilibrium. Ignoring changes in the active carbon may lead to a failure to address a new phenomenon that was previously considered to not occur in mature forests. For instance, the mechanism of increasing biomass carbons in boreal, temperate and tropical old-growth forests12 and soil organic carbon in subtropical old-growth forests2 could be hardly addressed. Until now, no attention has been paid to the roles that active biomass carbon might play in mature forest ecosystem processes and no studies have investigated the directional changes of the biomass carbon in active organs and small individuals of mature forests.
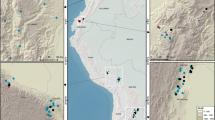
Here, we searched for directional changes in living biomass in mature forests located in the biomes of China's tropical forests and subtropical evergreen broadleaved forests (TEBFs) (Fig. 1) that compose more than 26% of China's terrestrial land area13. The main aim of this study was to partition the biomass of mature forests into active and inactive biomass carbon pools and understand their respective change trends.
Results
Trends of active and inactive biomass carbon pools
As shown in Fig. 2, among the 20 permanent plots in China's tropical/subtropical biome, the total biomass of entire stands did not display a significant increase (p = 0.132) from 1978 to 2012. In addition, the total biomass in branches and stems, arbor and DBH classes III and IV did not display significant increases (p>0.225). However, marked changes in the standing biomasses were observed in the leaves, roots, shrubs and small arbores and DBH classes I and II, in which the biomasses have all increased significantly (p<0.017) for more than three decades. Therefore, this group displayed a trend similar to that of the entire stand but with statistical significance. This difference may be due to the fact that the biomasses in the leaves, roots, shrubs and small arbor and DBH classes I and II amount to a small proportion of the entire stands. In the 20 permanent plots of mature forests, the average percentages of biomass in the leaves and roots, shrubs and small arbores and DBH classes I and II were only 20.6%, 7.2% and 5.9%, respectively. Therefore, even a significant change in the biomasses of these components would not affect the statistical significance of the overall trends in the entire stand.
Compound temporal trends in the biomass of the 20 permanently protected plots from 1978 to 2012.
The top row (panel (a)–(d)) is classified according to organs; the middle row (panel (e)–(h)) is classified according to functional groups; the bottom row (panel (i)–(l)) is classified according to DBH classes. For comparison, all original data (n = 99 in 20 plots) were standardised to the presented data with the averages and standard deviations in each plot being zero and one, respectively.
By partitioning the biomass into different organs for shrub, small arbor and arbor (Fig. 3), we found that the biomasses in the stems, branches, leaves and roots of arbor remained unchanged but the biomasses in all of the organs of both the shrubs and small arbores increased significantly. This result confirmed the finding14 that China's TEBF biomes are transitioning from cohorts of fewer and larger individuals to cohorts of a greater number of smaller individuals. The ecosystem may gradually be entering a lower non-equilibrium state because the shrub and small arbor groups would store less carbon than the arbor group.
Quantity of sequestrated biomass carbon in active organs and small individuals
Table 2 illustrates that yearly biomass increments in leaves and roots were 92 and 295 kg ha−1 yr−1, respectively; those in shrubs and small arbores were 26 and 394 kg ha−1 yr−1, respectively and those in DBH classes I and II were 139 and 273 kg ha−1 yr−1, respectively. In total, yearly biomass increments in organs, functional groups and DBH classes were 387, 420 and 412 kg ha−1 yr−1, respectively, averaging 406 kg ha−1 yr−1 in dry matter.
If we assume that the carbon content is 50%, the yearly increase in active carbon in the mature forests of China's TEBF biomes was 203 kg C ha−1 yr−1 over more than three decades, which yields a yearly sequestrated carbon amount of 0.012 Pg C yr−1 for the 5935.66×104 ha15 of natural forest in China's tropical and subtropical regions. This value is equivalent to the estimation of all of China's natural forests by Fang et al.7 during the same period. The value was estimated in accordance with the long-term observations in mature forests and should be considerably smaller than the amount of carbon that can be sequestrated by the natural forests of the area because most of the natural forests have not reached the ages of mature forests and are still in prior stages of development.
Discussion
Directional changes in biomass, composition and structure of China's TEBF biomes
We have previously found that China's TEBF biomes are transitioning from cohorts of fewer, larger individuals to ones with a higher number of smaller individuals14,16,17. This trend was characterized by an increased number of individuals and species for the shrub and small arbor groups and a decreased number of individuals and species for the arbor group14,17. In these studies, we elaborated the connections of the trends in structure and composition with global changes (global warming and precipitation pattern changes) and their consequent climate and soil drying.
In the present study, we further demonstrate that the reorganization in structure and composition has led the biomass carbon in leaves, roots and small individuals (shrubs and small arbores, DBH classes I and II) to a directional change. We did not find that the biomass carbon in the entire stands as well as in branches, stems and large individuals (inactive carbon) increase significantly. However, the biomass carbon in leaves, roots and small individuals (shrubs and small arbores, DBH classes I and II) (active carbon) have been accumulating rapidly over the past three decades. The results from some other studies can be taken as evidences of our finding. Piao et al.18 reported an increasing Normalized Difference Vegetation Index (NDVI) in the distribution area of China's TEBF biomes over the past decade. FACE experiments reported that litterfall increased with elevated atmospheric CO2 concentrations19,20,21,22,23 and predicted that changes in rainfall distribution patterns24 and temperature25 would also affect litterfall by altering leafing phenology26. The results imply that active biomass carbon was increased both in natural conditions and in FACE experiments.
Connections between active and inactive biomass carbon pools
Theoretically, the active biomass carbon pool plays the role of a transitional pool of photosynthesis products, which initiate all ecosystem processes. Partitioning the biomass carbon of a forest into two pools is important for addressing the mechanisms of non-equilibrium phenomena that are occurring in mature forests. Active biomass carbon is the outset of all biomass accumulation processes required to strengthen the connections between the changing environments and inactive carbon. The observed increase of inactive biomass carbon pools in intact African tropical forests is considered to be accompanied by a significant increase in the active biomass carbon pool6. In the present study, the biomasses in leaves and roots of both shrub and small arbor displayed significant increases, resulting in consistently significant increases in stems, branches and the entire functional groups of both shrub and small arbor. Correspondingly, for functional group of arbor, the biomasses in all organs and entire functional group did not display significant increases (Fig. 3). The results show that a positive relation between active and inactive biomass carbon pools in each functional group does exist. We suppose one of the reasons why the increased active biomass carbon pool (leaves, roots, or small individuals) in mature forests of China's TEBF biomes did not result in a significant increase in the inactive biomass carbon pool of the entire stands is because its biomass amounts to a small part. However, more studies on the nexuses between the two pools would be necessary in the future.
Demands for the nexuses between active biomass carbon and soil organic carbon
Soil organic carbon comes mainly from active biomass carbon. For aboveground and belowground processes, most of the active organs and small individuals complete their physiological functions in a few years, fall into the soil as fine litter and activate complex belowground processes27. In addition, after tens and hundreds of years, a small part of the inactive carbon may be artificially removed from the forest and thus lose its ecosystem functions; the remaining part of the inactive carbon will fall into the soil as coarse litter28. Fine litter may play a more important role in activating ecosystem processes than coarse litter. Studies have shown that litter with different sizes and organs decay at different rates29,30,31 and in turn influence the fraction of biomass carbon that move into the mineral soils32. Compared to coarse litter from stems and branches, fine litter that originates from leaves and roots would enhance the fractions of biomass carbon moving into mineral soils due to their higher nitrogen content and lower lignin content28,29,30,31,32,33,34,35. However, the effects of fresh carbon input to soil on soil carbon content has been highly controversial since 192636,37. Many studies have demonstrated a negative relationship between carbon input and soil carbon conservation26,37,38,39, which contradicts the traditional concepts10,11. Other studies still suggest the traditional conclusions35, depending on the vegetation types and environmental factors in wet tropical forests40,41,42. Notably, Cotrufo et al.35 confirmed a nexus between labile plant inputs and stable soil organic matter. Several observed increases in soil organic carbon in China's TEBF biomes have also been confirmed to be closely related to active carbon pools2,32,43. These studies have identified an urgent need to identify the states of active carbon pools in mature forests under changing environments to address the reactivation of belowground processes.
In the present study, we partitioned forest biomass carbon into active carbon and inactive carbon and found that the mature forests in China's TEBF biomes sequestrated carbon in active carbon pools instead of inactive carbon pools. Our finding provides direct evidence that mature forests have been shifted from their previous equilibrium by more labile plant inputs and have entered a new non-equilibrium state. These findings highlight the need to partition forest biomass carbon into the active and inactive pools as well as the need for additional studies on the nexus between the two pools and soil organic carbon.
Methods
Site description
The biomes of China's tropical forests and evergreen broadleaved forests (TEBFs) are distributed in the area from 21°N to 31°N in latitude and 101°E to 122°E in longitude. As the climax vegetation in this climate, TEBF dominates the direction in natural succession and recovery of all forests in the region13. At elevations higher than 2600 m, the original types of vegetation are Abies fabri (Mast) and mountain moss. Under the combined effects of the subtropical convergence and the uplift of the Himalayas, the monsoon climate prevails in this region, with a mean annual precipitation (MAP) of 1300–2000 mm, with nearly 80% falling in the wet season (April-September) and the other 20% falling in the dry season (October-March). The mean annual temperature (MAT) and relative humidity are 15.0–21.5°C and 78%, respectively. The environments in these plots differ significantly, as shown in Table S1.
The Chinese Academy of Sciences (CAS) and the Ministry of Science and Technology of P. R. China have arranged a series of field research stations across the region since the 1970s. Six of these field research stations (Tiantongshan TTS, Dinghushan DHS, Huitong HT, Gonggashan GGS, Xishuangbanna XSBN and Ailaoshan ALS) have a total of 20 permanent monitoring plots (TTK, TTM, DH1, DH2, DH3, DH4, DH5, HTK, GG1, GG2, GG3, GG4, BN1, BN2, BN3, BN4, BN5, ALZ and ALM) in the mature forests (Fig. 1, Table S1). When selecting permanent plots of matured forests, two procedures were involved to ascertain the plot's representativeness. First, the vegetation community structure and the composition of optional plots were studied to demonstrate that the forests were indeed mature. Second, various data were collected to demonstrate that the forest ecosystems had not been subject to artificial management and natural catastrophic events (e.g., landslides, typhoon, forest fires) for a sufficient period of time. Specifically, no trace of earlier management practices and no natural catastrophic events, including charcoal in the soils and tree stumps with fire scars or footprints of landslides and typhoons, could be observed. Moreover, soil profiles in the entire plot must be integrated and undisturbed. Regularly repeated censuses have been conducted since the establishment of each permanent plot using a unified methodology that was formulated by the Chinese Ecosystem Research Network.
Community surveys
All individuals with a diameter at breast height (DBH at 1.3 m above the ground) of at least 2 cm and a height of at least 1.5 m in the permanent sample plots were labelled in the first survey. Any newly recruited individuals who met these criteria were also labelled in the subsequent surveys. Each individual was assigned a unique number so that all individuals were accurately traced. During each survey, we identified the species of each labelled individual and measured the DBH and height.
Calculation of biomass
Forest biomasses were calculated using allometric equations that were established for each of the six stations. All of the allometric equations were subdivided into various organs (leaf, branch, stem and root)44,45,46,47,48,49.
Data standardisation
All of the calculated biomass data in each plot were standardised as follows:

where  is the standardised datum that correspond the original datum
is the standardised datum that correspond the original datum (dimensionless); i represents the 20 plots (i = TTK, TTM, DH1, DH2, DH3, DH4, DH5, DH6, HTK, GG1, GG2, GG3, GG4, BN1, BN2, BN3, BN4, BN5, ALZ and ALM); j is the census year from 1978 to 2012;
(dimensionless); i represents the 20 plots (i = TTK, TTM, DH1, DH2, DH3, DH4, DH5, DH6, HTK, GG1, GG2, GG3, GG4, BN1, BN2, BN3, BN4, BN5, ALZ and ALM); j is the census year from 1978 to 2012;  is the mean of the measurement values from 1978 to 2012 for plot i; and
is the mean of the measurement values from 1978 to 2012 for plot i; and  is the standard deviation of the measurement values from 1978 to 2012 for plot i.
is the standard deviation of the measurement values from 1978 to 2012 for plot i.
Data compiling
All species were classified into one of three functional groups (arbor, small arbor and shrub) according to the “Flora of China” records50, which was based on genetic characteristics of a given species. Hence, if a particular species belonged to shrub, small arbor, or arbor, for example, both its juvenile and mature individuals were given the same functional group classification. All individuals were also classified into one of four DBH classes (I: 2≤DBH≤5 cm; II: 5<DBH≤10 cm; III: 10<DBH≤20 cm and IV: DBH>20 cm). We analysed all of the data according to the three hierarchies: I organ or functional group or DBH class → II plot → III survey year. Data were standardised in each plot and the standardised data across all plots were subsequently analysed for statistical trends.
Statistical analysis
A simple linear regression model was used to analyse statistical trends for the standardised biomass data of the 20 plots. A linear mixed-effects model (LMM) with random effects was applied to the original biomass data to obtain the yearly accumulated biomasses.
References
Odum, E. P. The strategy of ecosystem development. Science 164, 262–270 (1969).
Zhou, G. Y. et al. Old-growth forests can accumulate carbon in soils. Science 314, 1417 (2006).
Phillips, O. L. et al. Changes in the carbon balance of tropical forests: Evidence from long-term plots. Science 282, 439–442 (1998).
Chave, J. et al. Assessing evidence for a pervasive alteration in tropical tree communities. PLoS Biol. 6, 0455–0462 (2008).
Luyssaert, S. et al. Old-growth forests as global carbon sinks. Nature 455, 213–215 (2008).
Lewis, S. L. et al. Increasing carbon storage in intact African tropical forests. Nature 457, 1003–1006 (2009).
Fang, J. Y., Chen, A. P., Peng, C. H., Zhao, S. Q. & Ci, L. J. Changes in forest biomass carbon storage in China between 1949 and 1998. Science 292, 2320–2322 (2001).
Sayer, E. J., Tanner, E. V. J. & Cheesman, A. W. Increased litterfall changes fine root distribution in a moist tropical forest. Plant Soil 281, 5–13 (2006).
Högberg, P. et al. High temporal resolution tracing of photosynthate carbon from the tree canopy to forest soil microorganisms. New Phytol. 177, 220–228 (2008).
Karlen, D. L. & Cambardella, C. A. Conservation strategies for improving soil quality and organic matter storage. Structure and Organic Matter Storage in Agricultural Soils Carter, R. & Stewart, B. A. (ed.), 395–420 (CRC Press, Boca Raton, 1996).
Parton, W. J., Ojima, D. S. & Schimel, D. S. Models to evaluate soil organic matter storage and dynamics. Structure and Organic Matter Storage in Agricultural Soils. Carter, R. & Stewart, B. A. (ed.), 420–448 (CRC Press, Boca Raton, 1996).
Muller-Landau, H. C. Sink in the African jungle. Nature 457, 969–970 (2009).
The editorial board of vegetation of China. Vegetation of China 1 (Science Press, Beijing, China, 1980).
Zhou, G. Y. et al. Substantial reorganization of China's tropical and subtropical forests: based on the permanent plots. Glob. Change Biol. 20, 240–250 (2014).
The editorial board of Reports on China's Forest Resources. Reports on China's Forest Resources-The Seventh National Survey on Forest Resources. (State Forestry Administration, PR China, 2009).
Zhou, G. Y. et al. Quantifying the hydrological responses to climate change in an intact forested small watershed in Southern China. Glob. Change Biol. 17, 3736–3746 (2011).
Zhou, G. Y. et al. A climate change-induced threat to the ecological resilience of a subtropical monsoon evergreen broad-leaved forest in Southern China. Glob. Change Biol. 19, 1197–1210 (2013).
Piao, S. L. et al. Interannual variations of monthly and seasonal normalized difference vegetation index (NDVI) in China from 1982 to 1999. J. Geophys. Res. 108 (2003).
DeLucia, E. H. et al. Net primary production of a forest ecosystem with experimental CO2 enrichment. Science 284, 1177–1179 (1999).
Allen, A. S. et al. Effects of free-air CO2 enrichment (FACE) on belowground processes in a Pinus taeda forest. Ecol. Appl. 10, 437–448 (2000).
Finzi, A. C., Allen, A. S., DeLucia, E. H., Ellsworth, D. S. & Schlesinger, W. H. Forest litter production, chemistry and decomposition following two years of free-air CO2 enrichment. Ecology 82, 470–484 (2001).
Schlesinger, W. H. & Lichter, J. Limited carbon storage in soil and litter of experimental forest plots under increased atmospheric CO2 . Nature 411, 466–469 (2001).
Zak, D. R., Holmes, W. E., Finzi, A. C., Norby, R. J. & Schlesinger, W. H. Soil nitrogen cycling under elevated CO2: a synthesis of forest FACE experiments. Ecol. Appl. 13, 1508–1514 (2003).
Zhang, X. et al. Detection of human influence on twentieth-century precipitation trends. Nature 448, 462–465 (2007).
Raich, J. W., Russell, A. E., Kitayama, K., Parton, W. J. & Vitousek, P. M. Temperature influences carbon accumulation in moist tropical forests. Ecology 87, 76–87 (2006).
Sayer, E. J., Powers, J. S. & Tanner, E. V. J. Increased litterfall in tropical forests boosts the transfer of soil CO2 to the atmosphere. PLoS One 2, e1299 (2007).
Zhou, G. Y. et al. Litterfall production along successional and altitudinal gradients of subtropical monsoon evergreen broadleaved forests in Guangdong, China. Plant Ecol. 188, 77–89 (2007).
Yang, F. F. et al. Dynamics of coarse woody debris and decomposition rates in an old-growth forest in lower tropical China. For. Ecol. Manage. 259, 1666–1672 (2010).
Zhang, D. Q., Hui, D. F., Luo, Y. Q. & Zhou, G. Y. Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors. J. Plant Ecol. 1, 85–93 (2008).
Zhou, G. Y. et al. Factors influencing leaf litter decomposition: an intersite decomposition experiment across China. Plant Soil 311, 61–72 (2008).
Makkonen, M. et al. Highly consistent effects of plant litter identity and functional traits on decomposition across a latitudinal gradient. Ecol. Lett. 15, 1033–1041 (2012).
Huang, Y. H. et al. Controls of litter quality on the carbon sink in soils through partitioning the products of decomposing litter in a forest succession series in South China. For. Ecol. Manage. 261, 1170–1177 (2011).
Bruckman, V. J., Yan, S., Hochbichler, E. & Glatzel, G. Carbon pools and temporal dynamics along a rotation period in Quercus dominated high forest and coppice with standards stands. For. Ecol. Manage. 262, 1853–1862 (2011).
Gul, S., Whalen, J. K., Ellis, B. E. & Mustafa, A. F. Influence of plant residue chemistry on soil CO2-C production: A study with Arabidopsis thaliana cell wall mutants of KNAT7, MYB75 and CCR1. Pedobiologia 55, 349–356 (2012).
Cotrufo, M. F., Wallenstein, M. D., Boot, C. M., Denef, K. & Paul, E. The Microbial Efficiency- Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter? Glob. Change Biol. 19, 988–995 (2013).
Löhnis, F. Nitrogen avaibility of green manures. Soil Sci. 22, 253–290 (1926).
Fontaine, S., Bardoux, G., Abbadie, L. & Mariotti, A. Carbon input to soil may decrease soil carbon content. Ecol. Lett. 7, 314–320 (2004).
Högberg, P. et al. Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411, 789–792 (2001).
Gill, R. A. et al. Nonlinear grassland responses to past and future atmospheric CO2 . Nature 417, 279–282 (2002).
Li, Y. Q., Xu, M., Sun, O. J. & Cui, W. C. Effects of root and litter exclusion on soil CO2 efflux and microbial biomass in wet tropical forests. Soil Biol. Biochem. 36, 2111–2114 (2004).
Schaefer, D. A., Feng, W. T. & Zou, X. M. Plant carbon inputs and environmental factors strongly affect soil respiration in a subtropical forest of southwestern China. Soil Biol. Biochem. 41, 1000–1007 (2009).
Leff, J. W. et al. Experimental litterfall manipulation drives large and rapid changes in soil carbon cycling in a wet tropical forest. Glob. Change Biol. 18, 2969–2979 (2012).
Tang, X. L. et al. Different patterns of ecosystem carbon accumulation between a young and an old-growth subtropical forest in Southern China. Plant Ecol. 212, 1385–1395 (2011).
Cheng, G. W. & Wang, G. X. Long-Term Data Sets of Monitoring and Researches on Chinese Ecosystems: Volume of Forest Ecosystem-Gonggashan Station, Sichuan province (1995–2006). (China Agriculture Press, Beijing, China, 2011).
Deng, X. B. & Tang, J. W. Long-Term Data Sets of Monitoring and Researches on Chinese Ecosystems: Volume of Forest Ecosystem-Xishuangbanna Station, Yunan province (1998–2006). (China Agriculture Press, Beijing, China, 2011).
Wang, S. L. Long-Term Data Sets of Monitoring and Researches on Chinese Ecosystems: Volume of Forest Ecosystem-Huitong Station, Hunan province (1960–2006). (China Agriculture Press, Beijing, China, 2011).
Wang, X. H. Long-Term Data Sets of Monitoring and Researches on Chinese Ecosystems: Volume of Forest Ecosystem-Tiantongshan Station, Zhejiang province (1983–2009). (China Agriculture Press, Beijing, China, 2011).
Zhang, Q. M. Long-Term Data Sets of Monitoring and Researches on Chinese Ecosystems: Volume of Forest Ecosystem-Dinghushan Station, Guangdong province (1998–2008). (China Agriculture Press, Beijing, China, 2011).
Zhang, Y. P. & Liu, Y. H. Long-Term Data Sets of Monitoring and Researches on Chinese Ecosystems: Volume of Forest Ecosystem-Ailaoshan Station, Yunan province (2003–2007). (China Agriculture Press, Beijing, China, 2011).
The editorial board of flora of China. Flora of China. (Science Press. Beijing, China, 2004).
Acknowledgements
Funding for the study was provided by the XDA05050200 project. The study is administrated by the Chinese Ecosystem Research Network (CERN). We are grateful to all the researchers and students who were involved in the related field surveys. The PIs of the six forest stations are Deqiang Zhang (DHS), Min Cao (XSBN), Genxu Wang (GGS), Xihua Wang (TTS), Silong Wang (HT) and Yiping Zhang (ALS).
Author information
Authors and Affiliations
Contributions
Y.X. analysed the data and wrote the manuscript. G.Z. designed the study and proposed the scientific hypothesis. Q.Z. compiled the data. W.W. performed the correlation analysis and developed the figures. S.L. compiled the data for Dinghushan.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Increasing active biomass carbon may lead to a breakdown of mature forest equilibrium
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Xiao, Y., Zhou, G., Zhang, Q. et al. Increasing active biomass carbon may lead to a breakdown of mature forest equilibrium. Sci Rep 4, 3681 (2014). https://doi.org/10.1038/srep03681
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep03681
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.