Abstract
In order to understand and rationally construct homochiral self-assembled structures from racemic molecules, two novel crystalline metal-organic frameworks with chiral cavities were developed. The homochirality of the layers in both MOFs was achieved by forming strong coordinate bonds between the C3-symmetric cyclotriveratrylene and Zn4O(CO2)6 cluster. By changing weak π-π interactions between organic building blocks, the achiral assembly of ZnCTV-1 was successfully transformed into a chiral assembly in ZnCTV-2. This study demonstrated a possible route for designing the synthesis of chiral MOF through weak interactions.
Similar content being viewed by others
Introduction
Chirality is a common phenomenon in nature and represents the evolution of matters towards more complicated cases. Many chiral systems achieve their functions through the interaction with other chiral molecules or the environment (chiral recognition or chiral assembly)1,2,3. However, the rules controlling (a)chiral interactions, such as chiral symmetry breaking, self-resolution, supramolecular chirality formed by achiral molecular aggregation, etc. remain relatively poorly understood. Therefore, the understanding and rational construction of chiral structures from achiral or racemic molecules are still challenging4,5.
Metal-organic frameworks (MOFs), an important class of self-assembled materials, built by organic linkers and metal ions or clusters have attracted great attention over the past decades due to the structural diversity and outstanding properties6,7,8,9,10,11,12,13,14,15,16,17,18,19,20. Chiral MOFs are more attractive because of their additional properties for asymmetric catalysis, entiomorphous selectivity, chiral molecular imaging, etc14,15,16,17,18. To prepare homochiral MOF materials, three complimentary approaches have been widely used19: direct synthesis from enantiopure ligands or via self-resolution process, chiral-templated synthetic procedures and post-synthetic modification approaches. Among these, spontaneous resolution during crystallization is of higher interest.
Cyclotriveratrylene (CTV) is a rigid cyclic macromolecule with a shallow electron-rich cavity20,21,22. New progress in the domain of macrocyclic CTV-based hosts includes applications in material science, sensing and separations23,24,25,26,27,28. Owing to a pyramidal shape and convergent binding modes, CTV itself and CTV-based ligands are capable of forming coordination complexes with group one elements and transition metals29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44. Hardie et al. conducted excellent works on pyridyl decorated CTV derivatives that form discrete or infinite metallo-supramolecular assemblies33,34,35,36,37,38. When it comes to carboxylic acid decorated CTV ligands, only a few structures have been reported39,40,41,42,43,44 and their transition metal complexes are even rarer42,43,44. We recently reported the first CTV based 1D ternary independent nanotubular framework with permanent porosity44. In this work, C3-symmetric tritopic corner ligands tri-(4- carboxyl)-trimethoxycyclobenzylebe (H3L1) and tri-(4-carboxy-phenyl)-trimethoxy- cyclobenzylebe (H3L2) were used for the construction of chiral MOFs (Fig. 1a). As far as we know, there is no CTV-based infinite coordination polymer with chiral cavities reported until now.
Here, six-coordinated octahedral Zn4O(CO2)6 clusters were selected as inorganic nodes due to a relative easy formation as well as good thermal stability. According to the principles of reticular chemistry, (6,3)-coordinated networks are expected. Yaghi et al. have reported two kinds of 3D (6,3)-coordinated networks (known as pyr45 and qom46 topology) with flat three-connected organic linkers and Zn4O(CO2)6 clusters, which show ultrahigh porosity than most MOFs. While, due to the concave and bowl-shape structure of CTV type ligands, different topologies are formed as we will discuss below.
Results
Synthesis and crystal structure of ZnCTV-1
A preliminary experiment resulted in the formation of ZnCTV-1, which is synthesized from the solvothermal reaction of Zn(NO3)2·6H2O and H3L1 in N,N′-dimethylformamide (DMF) at 95°C (Fig. 1b). The colourless hexagonal plate crystals of ZnCTV-1 are insoluble in water and common organic solvents (DMF, alcohol, THF, etc), elemental analysis gave a formula of [Zn4O(L1)2]·DMF·EtOH·H2O. Single crystal X-ray diffraction analysis showed that ZnCTV-1 is a stacking of 2D (6,3)-coordinated layers in the space group of P31c. Each layer can be considered as a fused honeycomb bilayer in eclipsed mode shown in Fig. 2a & 2b. In each layer, face-to-face arranged ligands of the same enantiomer link to Zn4O(CO2)6 clusters through their carboxylates, forming rigid chiral capsules with C3 symmetry and a cavity volume of 167 Å3. The homochirality in each bilayer is due to symmetry restriction and the chirality was transferred from one ligand to another through the rigid Zn4O(CO2)6 cluster. Unfortunately, the adjacent bilayers are symmetrically related by the c-glide and contain the opposite enantiomers (Fig. 2c).
Based on the analysis of ZnCTV-1, we could devise two possible ways to obtain a chiral MOF. The first consists in using a homochiral CTV ligand to exclude the formation of the layers with the opposite chirality. However, with the present solvothermal synthetic conditions, ligand molecules could easily flip to their enantiomers during the reaction process47. A new synthetic route with milder conditions is under investigation. The alternative route is to keep the homochiral layer but modify the interaction between layers to assemble the homochiral layers selectively In ZnCTV-1, the main interaction between layers is the π-π interaction in a parallel-displaced mode with a plane distance of about 3.2 Å (Supplementary Fig. S2). In order to modify the possible π-π interaction, our strategy is to insert a phenyl group between the CTV-skeleton and the carboxylate group with the H3L2 shown in Fig. 1.
Synthesis and crystal structure of ZnCTV-2
Similar solvothermal reactions of ZnCl2 and H3L2 in N,N′-diethylformamide (DEF) produced beautiful octahedral crystals, ZnCTV-2 (Fig. 1c), with a yield of 86%. The product was formulated as Zn4O(L2)2·13DEF·3H2O on the basis of elemental analysis. Single crystal X-ray diffraction revealed also a layered (6,3)-connected framework but with the topology shown in Fig. 3b with space group R32. This layer can also be considered as a fused honeycomb bilayer but in a staggered mode (Fig. 3a & 3b). Although the geometry restriction here is weaker than that in ZnCTV-1 structure due to the longer CTV arms, all CTV molecules in each layer still have the same chirality as those in ZnCTV-1. However, in stark contrast with ZnCTV-1, the ligands of adjacent layers in ZnCTV-2 have the same chirality and the whole structure is in a chiral space group (Fig. 3c). Note that due to the racemic nature of the reactant, the whole batch of materials are considered as a mixture of homochiral left and right-handed crystals.
Weak interactions in ZnCTV-2
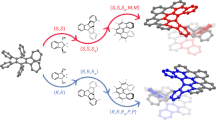
To understand the formation of homochiral ZnCTV-2, the interaction between the honeycomb bilayers were examined carefully. There are two types of bilayer interfaces. At z = 0, two CTV molecules are symmetry related by the 2-fold rotation and their two pairs of phenyl groups have a plane distance about 3.4 Å, showing typical π-π interactions in a parallel-displaced mode (Fig. 4a & Supplementary Fig. S3). At z = 0.5, two CTV molecules are also symmetry related by the 2-fold rotation, while the two pairs of phenyl groups have a typical π-π interaction in a T-shaped mode48 with a C-Centroid distance of about 3.9Å (Supplementary Fig. S3). Thus, in both cases, the interactions are about twice as strong as those in ZnCTV-1 (only one pair of π-π interaction). This may explain why the ZnCTV-2 crystals are much thicker than ZnCTV-1 along the c-axis. Moreover, as we expected, the stronger interaction between the bilayers in ZnCTV-2 may increase the chiral recognition and result in a spontaneous self-sorting process of CTV molecules during the crystallization. To confirm this, two structure models were built with racemic CTVs in adjacent bilayers and it turns out that due to the long CTV arms, the π-π interaction between layers becomes much weaker than that of ZnCTV-2 as shown in Fig. 4 (see also Supplementary Fig. S4–S7 for all possible models). A structure model with layers the same as ZnCTV-1 was also built (see Supplementary Fig. S6 & S7) but no efficient π-π interaction or other hydrophobic interactions between layers were found. Thus, we can conclude that by elongation of the CTV arms with a phenyl group, the inter layer π-π interactions became more selective and finally resulted in the chiral structure of ZnCTV-2.
The π-π interactions of two CTV ligands from adjacent layers.
(a), The π-π interactions of two homochiral ligands from adjacent layers in a parallel-displaced mode in the real structure of ZnCTV-2. (b) and (c), Two possible models with racemic organic linkers. Homochiral ZnCTV-2 contains more π-π interaction sites than the racemic models. Two adjacent interacting phenyls are shown in same color.
Stability and porosity
The stability and porosity were studied by thermal gravimetric analysis (TGA) and N2 gas adsorption. Although ZnCTV-1 has a solvent void volume of about 577 Å3 (20% of the cell volume) from the SQUEEZE calculation in PLATON, the void is not accessible since the framework decomposed as soon as the guest molecule started releasing at ca. 340°C (Supplementary Fig. S9 & S11), similar to other previously observed layered frameworks without permanent porosity49. Solvent exchange process and evacuation also failed in emptying the pores because of the small windows of the capsule. The total solvent accessible void volume of ZnCTV-2 is 15573 Å3 (63% of the cell volume) (Fig. 5), where guest molecules are highly disordered and cannot be located by X-ray diffraction. According to TGA, the weight loss of 44% below 270°C corresponds to the release of 13 DEF molecules together with three water molecules and the framework is stable up to 410°C. The PXRD pattern of the desolvated sample reveals that, upon guests exchange and evacuation, the desolvated materials have much lower crystallinity and its structure cannot be obtained (Supplementary Fig. S10 & S12). N2 gas adsorption isotherms of desolvated ZnCTV-2 at 77K shows a reversible type I behaviour without hysteresis upon desorption, indicating an open framework with accessible microporosity (Supplementary Fig. S13–S16).
Discussion
CTV analogues are a type of macrocyclic molecule with convergent binding modes, which can be easily functionalized to construct amazing structures. By using reticular chemistry, we prepared two novel crystalline MOFs with chiral cavities based on C3-symmetric macrocyclic CTV ligands and the octahedral Zn4O(CO2)6 clusters. We showed our strategy to understand and to construct homochiral MOFs from racemic C3-symmetrical linkers. In the layer, the rigid Zn4O(CO2)6 cluster guides the selection of enantiomers through strong coordination bonds, while between layers, the relatively weak π-π interaction plays a great role in directing the formation of chiral MOFs. This approach contributes a new guidance in the construction of chiral functional materials.
Methods
Materials
All reagents and solvents used in synthetic studies were commercially available and used as supplied without further purification unless stated otherwise. Ligand H3L1 and H3L2 were synthesized according to the method as shown in Supplementary or according to the literature procedure32,44.
Synthesis of ZnCTV-1
A mixture of H3L1 (9 mg, 0.018 mmol) and Zn(NO3)2·6H2O (19 mg, 0.064 mmol) was dissolved in a 20 mL Teflon vial containing 1 mL DMF, 1 mL ethanol and 0.5 mL H2O. The vial was sealed and heated at 95°C for 48 h. The resulting colorless hexagonal plate crystals were collected and washed with DMF and ethanol, dried in air overnight (10 mg, 77%). Elemental analysis (%): calcd. for [Zn4O(L1)2]·DMF·EtOH·H2O (C59H57NO22Zn4): C, 50.84; H 4.12; N 1.00; found: C, 50.84; H, 4.14; N 1.06.
Synthesis of ZnCTV-2
A mixture of H3L2 (10 mg, 0.014 mmol) and ZnCl2 (11.5 mg, 0.084 mmol) was dissolved in a 20 mL Teflon vial containing 1 mL DEF, 1 mL ethanol and 0.2 mL H2O. The vial was sealed and heated at 105°C for 48 h. The resulting small colorless octahedron like crystals were collected, washed with DEF dried in air overnight (18 mg, 86%). Elemental analysis (%): calcd. for Anal. Calcd. for [Zn4O(L2)2]·13DEF·3H2O (C155H215N13O35Zn4): C, 60.40; H, 7.03; N, 5.91; found: C, 60.16; H, 6.48; N, 6.08.
References
Moriuchi, T. & Hirao, T. Design of ferrocene-dipeptide bioorganometallic conjugates to induce chirality-organized structures. Acc. Chem. Res. 43, 1040–1051 (2010).
Yashima, E. Chiral and chirality discrimination on helical polyacetylenes. Anal. Sci. 18, 3–6 (2002).
Ddachi, K., Chayama, K. & Watarai, H. Control of optically active structure of thioether-phthalocyanine aggregates by chiral Pd(II)-BINAP complexes in toluene and at the toluene/water interface. Chirality 18, 599–608 (2006).
Pérez-García, L. & Amabilino, D. B. Spontaneous resolution, whence and whither: from enantiomorphic solids to chiral liquid crystals, monolayers and macro- and supra-molecular polymers and assemblies. Chem. Soc. Rev. 36, 941–967 (2007).
Pérez-García, L. & Amabilino, D. B. Spontaneous resolution under supramolecular control. Chem. Soc. Rev. 31, 342–356 (2002).
Kitagawa, S., Kitaura, R. & Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 43, 2334–2375 (2004).
Ferey, G. Hybrid porous solids: past, present, future. Chem. Soc. Rev. 37, 191–214 (2008).
Horike, S., Shimomura, S. & Kitagawa, S. Soft porous crystals. Nat. Chem. 1, 695–704 (2009).
Deng, H. et al. Multiple functional groups of varying ratios in metal-organic frameworks. Science 327, 846–850 (2010).
Lee, J. Y. et al. Metal-organic framework materials as catalysts. Chem. Soc. Rev. 38, 1450–1459 (2009).
Chen, B., Xiang, S. -C. & Qian, G. -D. Metal-organic frameworks with functional pores for recognition of small molecules. Acc. Chem. Res. 43, 1115–1124 (2010).
Britt, D., Furukawa, H., Wang, B., Glover, T. G. & Yaghi, O. M. Highly efficient separation of carbon dioxide by a metal-organic framework replete with open metal sites. Proc. Natl Acad. Sci. USA 106, 20637–20640 (2009).
Xiang, S. et al. Rationally tuned micropores within enantiopure metal-organic frameworks for highly selective separation of acetylene and ethylene. Nat. commun. 2, 204–210 (2011).
Ma, L., Abney, C. & Lin, W. Enantioselective catalysis with homochiral metal-organic frameworks. Chem. Soc. Rev. 38, 1248–1256 (2009).
Bernhard, S., Takada, K., Díaz, D. J., Abruña, H. D. & Mürner, H. Enantiomerically pure chiral coordination polymers: synthesis, spectroscopy and electrochemistry in solution and on surfaces. J. Am. Chem. Soc. 123, 10265–10271 (2001).
Xie, S.-M., Zhang, Z.-J., Wang, Z.-Y. & Yuan, L.-M. Chiral metal-organic frameworks for high-resolution gas chromatographic separations. J. Am. Chem. Soc. 133, 11892–11895 (2011).
Dang, D., Wu, P., He, C., Xie, Z. & Duan, C. Homochiral metal-organic frameworks for heterogeneous asymmetric catalysis. J. Am. Chem. Soc. 132, 14321–14323 (2010).
Yoon, M., Srirambalaji, R. & Kim, K. Homochiral metal-organic frameworks for asymmetric heterogeneous catalysis. Chem. Rev. 112, 1196–1231 (2012).
Liu, Y., Xuan, W. & Cui, Y. Engineering homochiral metal-organic frameworks for heterogeneous asymmetric catalysis and enantioselective separation. Adv. Mater. 22, 4112–4135 (2010).
Collet, A. Cyclotriveratrylenes and crypyophanes. Tetrahedron 43, 5725–5759 (1987).
Collet, A., Dutasta, J. P., Lozach, B. & Canceill, J. Cyclotriveratrylene and cryptophanes: their synthesis and applications to host-guest chemistry and to the design of new materials. Top. Curr. Chem. 165, 104–129 (1993).
Hardie, M. J. Recent advances in the chemistry of cyclotriveratrylene. Chem. Soc. Rev. 39, 516–527 (2010).
Yu, J.-T., Chen, Z., Sun, J., Huang, Z.-T. & Zheng, Q.-Y. Cyclotricatechylene based porous crystalline materials: synthesis and applications in gas storage. J. Mater. Chem. 22, 5369–5373 (2012).
Kubo, Y., Yoshizumi, W. & Minami, T. Development of chemical stimuli-responsive organogel using boronate ester-substituted cyclotricatechylene. Chem. Lett. 37, 1238–1239 (2008).
Schröder, L., Lowery, T. J., Hilty, C., Wemmer, D. E. & Pines, A. Molecular imaging using a targeted magnetic resonance hyperpolarized biosensor. Science 314, 446–449 (2006).
Shi, Y.-Y., Sun, J., Huang, Z.-T. & Zheng, Q.-Y. Crystalline self-assembly of a bowl-like cyclotriguaiacylene derivative with alcohol/phenols by hydrogen bonding and C-H… π interactions: the self-inclusion extended organic frameworks. Cryst. Growth & Des. 10, 314–320 (2010).
Wang, L., Wang, G.-T., Zhao, X., Jiang, X.-K. & Li, Z.-T. Hydrogen bonding-directed quantitative self-assembly of cyclotriveratrylene capsules and their encapsulation of C60 and C70 . J. Org. Chem. 76, 3531–3535 (2011).
Huerta, E. et al. Selective binding and easy separation of C70 by nanoscale self-assembled capsules. Angew. Chem. Int. Ed. 46, 202–205 (2007).
Hardie, M. J., Ahmad, R. & Sumby, C. J. Network structures of cyclotriveratrylene and its derivatives. New J. Chem. 29, 1231–1240 (2005).
Abrahams, B. F., FitzGerald, N. J., Hudson, T. A., Robson, R. & Waters, T. Closed and open clamlike structures formed by hydrogen-bonded pairs of cyclotricatechylene anions that contain cationic “meat”. Angew. Chem. Int. Ed. 48, 3129–3132 (2009).
Abrahams, B. F., FitzGerald, N. J. & Robson, R. Cages with tetrahedron-like topology formed from the combination of cyclotricatechylene ligands with metal cations. Angew. Chem. Int. Ed. 49, 2896–2899 (2010).
Zhong, Z., Ikeda, A., Shinkai, S., Sakamoto, S. & Yamaguchi, K. Creation of novel chiral cryptophanes by a self-assembling method utilizing a pyridyl-Pd(II) interaction. Org. Lett. 3, 1085–1087 (2001).
Westcott, A., Fisher, J., Harding, L. P., Rizkallah, P. & Hardie, M. J. Self-assembly of a 3-D triply interlocked chiral [2]catenane. J. Am. Chem. Soc. 130, 2950–2951 (2008).
Ronson, T. K. et al. Tripodal 4-pyridyl-derived host ligands and their metallo-supramolecular chemistry: stella octangula and bowl-shaped assemblies. Inorg. Chem. 49, 675–685 (2010).
Ronson, T. K., Fisher, J., Harding, L. P. & Hardie, M. J. Star-burst prisms with cyclotriveratrylene-type ligands: a [Pd6L8]12+ stella octangular structure. Angew. Chem. Int. Ed. 46, 9086–9088.
Ronson, T. K. et al. Stellated polyhedral assembly of a topologically complicated Pd4L4 “Solomon cube”. Nat. Chem. 1, 212–216 (2009).
Ronson, T. K. & Hardie, M. J. Extended 36 and 63 arrays of capsule motifs using ligand tris{4-(3-pyridyl)phenylester} cyclotriguaiacylene. CrystEngComm 1731–1734 (2008).
Sumby, C. J., Fisher, J., Prior, T. J. & Hardie, M. J. Tris (pyridylmethylamino)cyclotriguaiacylene cavitands: an investigation of the solution and solid-state behaviour of metallo-supramolecular cages and cavitand-based coordination polymers. Chem. Eur. J. 12, 2945–2959 (2006).
Zhan, H.-Q., Jiang, X.-K. & Li, Z.-T. Supramolecular complexation behavior of novel cyclotriveratrylene derivatives with benzoate pendants with C60 . Chin. J. Chem. 19, 147–153 (2001).
Roesky, C. E. O. et al. A new cryptophane receptor featuring three endo-carboxylic acid groups: synthesis, host behavior and structural study. Chem. Eur. J. 9, 1104–1112 (2003).
Le Gac, S., Luhmer, M., Reinaud, O. & Jabin, I. Self-assembly via ionic interactions of calix[6]arene-based receptors displaying remarkable host–guest properties toward neutral guests. Tetrahedron 63, 10721–10730 (2007).
Mough, S. T. & Holman, K. T. A soft coordination polymer derived from container molecule ligands. Chem. Commun. 1407–1409 (2008).
Ronson, T. K., Nowell, H., Westcott, A. & Hardie, M. J. Bow-tie metallo-cryptophanes from a carboxylate derived cavitand. Chem. Commun. 176–178 (2011).
Yu, J.-T., Sun, J., Huang, Z.-T. & Zheng, Q.-Y. A novel 1D independent metal–organic nanotube based on cyclotriveratrylene ligand. CrystEngComm 14, 112–115 (2012).
Chae, H. K., Kim, J., Friedrichs, O. D., O'Keeffe, M. & Yaghi, O. M. Design of frameworks with mixed triangular and octahedral building blocks exemplified by the structure of [Zn4O(TCA)2] having the pyrite topology. Angew. Chem. Int. Ed. 42, 3907–3909 (2003).
Chae, H. K. et al. A route to high surface area, porosity and inclusion of large molecules in crystals. Nature 427, 523–527 (2004).
Collet, A. & Gabard, J. Optically active (C3)-cyclotriveratrylene-d9. Energy barrier for the “crown to crown” conformational interconversion of its nine-membered-ring system. J. Org. Chem. 45, 5400–5401 (1980).
Hunter, C. A. & Sanders, J. K. M. The nature of π-π interactions. J. Am. Chem. Soc. 112, 5525–5534 (1990).
Humphrey, S. M., Allan, P. K., Oungoulian, S. E., Ironside, M. S. & Wise, E. R. Metal-organophosphine and metal-organophosphonium frameworks with layered honeycomb-like structures. Dalton Trans. 2298–2305 (2009).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (20932004), the Major State Basic Research Development Program of China (2011CB932501 & 2013CB933402) and the Chinese Academy of Sciences.
Author information
Authors and Affiliations
Contributions
J.-T.Y., J.S., J.L., Z.-T.H. and Q.-Y.Z. designed the study, analyzed the data and wrote the paper. Y.-Y.S. conducted part of the synthetic work. J.S. and Q.-Y.Z. performed the crystal-structure analyses and constructed the models. J.-T.Y. performed the sorption measurements. All authors discussed the results and commented on the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
SI for ZNCTV paper
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Yu, JT., Shi, YY., Sun, J. et al. Spontaneous chiral resolution directed by symmetry restriction and π-π interaction. Sci Rep 3, 2947 (2013). https://doi.org/10.1038/srep02947
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep02947
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.