Abstract
We investigated the effects of extracts of Benifuuki (a tea cultivar that contains methylated catechins such as epigallocatechin-3-O-(3-O-methyl) gallate (EGCG3”Me)) in mice fed a high-fat/high-sucrose (HF/HS) diet. This tea cultivar was then compared with an extract of Yabukita (a popular tea cultivar that lacks methylated catechins). For 6 weeks, C57BL/6J mice were fed either HF/HS diet with or without tea extracts from tea cultivars, which contained almost identical ingredients except for methylated catechins (i.e., Yabukita (0.2% and 1%) or Benifuuki (0.2% and 1%) extract powders). Supplementation with Benifuuki 0.2% markedly lowered plasma levels of TG and NEFAs compared with mice supplemented with Yabukita 0.2%. The diet containing Benifuuki 1% decreased adipose tissue weights, liver TG and expression of lipogenic genes in the liver. These results suggested that Benifuuki had much greater lipid-lowering effects than Yabukita. Taken together, these data suggest that methylated catechins direct the strong lipid-lowering activity of Benifuuki.
Similar content being viewed by others
Introduction
Obesity is a metabolic disturbance resulting from an imbalance between fat synthesis (lipogenesis) and fat breakdown (oxidation). Obesity is prevailing at an alarming rate worldwide. Obesity is associated with an increased risk of cardiovascular disease1. Indeed, obesity-induced hypercholesterolaemia is correlated with coronary heart disease risk2.
Green tea (Camellia sinensis L.) is one of the most popular beverages in the world. It has been reported that daily consumption of green tea is associated with several important health benefits, including antioxidant, anti-carcinogenic, hypocholesterolemic and cardioprotective activities3,4,5,6. The beneficial properties of green tea are attributable to the abundant polyphenolic compounds (“catechins”) that it contains. The green-tea catechins include catechin (C), (−)-epicatechin (EC), (−)-epigallocatechin (EGC), (−)-epicatechin-3-gallate (ECG) and (−)-epigallocatechin-3-gallate (EGCG). Of these, EGCG is a predominant catechin and has several biological and pharmacological properties7,8,9,10,11,12,13,14,15,16. Several studies have reported the anti-obesity effects of green tea and EGCG17,18,19,20.
Benifuuki (Camellia sinensis L.) is a tea cultivar that was originally established by the National Institute of Vegetable and Tea Science (National Agriculture and Food Research Organization, Tokyo, Japan). Benifuuki is rich in methylated catechins such as epigallocatechin-3-O-(3-O-methyl) gallate (EGCG3”Me; ≈ 0.8–2.5% (w/w)), which is not present in Yabukita cultivar, the most popular green tea, which accounts for about 75% of green-tea products consumed in Japan. In vivo and clinical studies have shown that Benifuuki has strong anti-allergic effects21,22. Pharmacokinetic analyses confirmed that EGCG3”Me has a significantly higher absorption rate from the intestine and a lower rate of disappearance from the blood than EGCG23. Therefore, although the amount of EGCG3”Me in Benifuuki tea is about one-quarter that of EGCG, the blood concentrations of these two catechins after ingestion of Benifuuki tea are almost identical23. This is considered to be the main reason why Benifuuki has greater anti-allergic activity than Yabukita. The anti-allergic effects of Benifuuki have been reported, but studies describing the beneficial anti-obesity effects of Benifuuki have not.
The goals of the present study were to determine the effect of dietary Benifuuki on fat accumulation, lipid levels in the plasma and liver and hepatic gene expression in a HF/HS-induced model of obesity in mice.
Results
General observations
Benifuuki is rich in methylated catechins such as EGCG3”Me, which is not present in Yabukita, the common cultivar. To determine the effect of dietary Benifuuki on fat accumulation, lipid levels in the plasma and liver and hepatic gene expression in a HF/HS-induced model of obesity and metabolic syndrome in mice, mice were given Yabukita or Benifuuki extract (Table 1) as described.
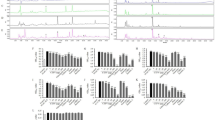
HF/HS feeding for 6 weeks significantly increased body weight and the weight of retroperitoneal adipose tissue compared with a control diet (Table 2). The groups fed Yabukita 1% and Benifuuki 1% had moderate decreased in body weight and weight of retroperitoneal adipose tissue compared with the HF/HS-fed group. The groups fed Yabukita 1% and Benifuuki 1% had a moderate decrease in body weight and weight of retroperitoneal adipose tissue compared with the HF/HS-fed group. The groups fed Benifuuki 1% had a moderate decrease in both rWAT and eWAT. However, the group fed Yabukita 1% had a moderate decrease in only eWAT. The cumulative energy intake did not significantly differ among the HF/HS group and the tea-extract groups except Benifuuki 0.2% group (Fig. 1).
Biochemical analyses of plasma
HF/HS feeding significantly elevated levels of total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and very-high density lipoprotein cholesterol (VLDL-C) (Table 2). A Benifuuki 1% diet significantly reduced levels of LDL-C and VLDL-C. Yabukita 1% as well as Benifuuki 0.2% and 1% diets significantly decreased plasma levels of aspartate aminotransferase (AST) and alanine transaminase (ALT). HF/HS feeding also elevated plasma levels of TG (Fig. 2A). A Yabukita 0.2% diet did not affect plasma levels of TG, whereas Yabukita 1% as well as Benifuuki 0.2% and 1% diets lowered plasma levels of TG to those seen in controls. Yabukita 1% as well as Benifuuki 0.2% and 1% diets lowered plasma levels of non-esterified fatty acid (NEFA) (Fig. 2B).
Liver lipids
HF/HS feeding for 6 weeks increased the content of liver TG. A Benifuuki 1% diet had a profound lowering effect upon liver levels of TG (Fig. 3).
DNA chip analyses
Among 194 genes associated with the metabolism of lipids and carbohydrates, we used data for the other 121 genes for analyses in the present study because the expressions of 73 of these genes were barely detected in liver samples (Supplementary Table S1 and S2). DNA chip analyses revealed that HF/HS, Yabukita and Benifuuki diets had a wide influence on mRNA expression. The diets affected the genes linked to energy production, redox regulation, defense against reactive oxygen species (ROS), the mitogen-activated protein kinase (MAPK) cascade, nuclear receptors, metabolism of energy and cholesterol and protein degradation. In particular, the expression of genes related to the metabolism of energy and cholesterol was influenced by the Benifuuki diet (Supplementary Table S1 and S2).
Expression of genes involved in the metabolism of lipids and cholesterol
We examined the expression of hepatic genes that regulate lipogenesis, beta-oxidation and cholesterol metabolism to explore the molecular mechanism underlying the hypolipidemic effect of a Benifuuki diet by real-time PCR. A Benifuuki diet decreased the expression of genes associated with lipogenesis such as sterol regulatory element binding protein-1c (SREBP-1c), acetyl-CoA carboxylase1 (ACC1), fatty acid synthase (FAS) and stearoyl-CoA desaturase1 (SCD1), compared with the HF/HS-fed group (Fig. 4). HF/HS feeding increased the expression of hepatic 3-hydroxy-3methyl-glutaryl-CoA reductase (HMGCR) and 3-hydroxy-3-methylglutaryl-CoA synthase (HMGCS), whereas Yabukita 1% and Benifuuki 1% diets lowered their expressions to those seen in controls. HF/HS did not affect the expression of cholesterol 7-alpha-monooxygenase (CYP7A1), whereas Yabukita 1% and Benifuuki 1% diets increased the expression of CYP7A1 mRNA (Fig. 5).
Effects of Yabukita and Benifuuki on mRNA levels of genes involved in lipid metabolism in liver.
The mRNA expression levels of genes involved in TG synthesis (SREBP-1c, ACC1, FAS and SCD1) and fatty acid metabolism (ACO and MCAD) in the liver of C57/BL6J mice fed diets containing HF/HS and Yabukita 1% or Benifuuki 1% extract powders. Values are means ± SEM, n = 6. Different letters are statistically different, p < 0.05.
Effects of Yabukita and Benifuuki on mRNA levels of genes involved in cholesterol metabolism in liver.
The mRNA expression levels of genes involved in cholesterol metabolism (HMGCR, HMGCS and CYP7A1) in C57/BL6J mice fed diets containing HF/HS and Yabukita 1% or Benifuuki 1% extract powders. Values are means ± SEM, n = 6. Different letters are statistically different, p < 0.05.
Discussion
In the present study, we showed that Benifuuki (a tea cultivar that contains methylated catechins) had a stronger lowering effect on plasma and hepatic TGs in HF/HS diet-fed mice than Yabukita (which lacks methylated catechins).
Mice fed Benifuuki 1% had reduced adipose tissue weights and liver TG content. Yabukita showed a less potent effect on TG content. These results suggested that Benifuuki had much greater hypolipidemic activity and anti-obesity effects than Yabukita. A marked difference in the total energy intake between HF/HS, Yabukita 1% and Benifuuki 1% groups was not observed. Therefore, various mechanisms leading to increased energy expenditure or lower fat deposition might be involved in the effects elicited by Benifuuki. Dietary HF/HS is a strong inducer of SREBP1-c, FAS, SCD1 and other enzymes regulating hepatic lipogenesis and produces a pronounced elevation of hepatic TG concentrations with an increase in plasma TG24,25,26,27,28,29. Benifuuki downregulated the expression of lipogenic enzyme genes such as SREBP-1c, ACC1, FAS and SCD1 in the liver. Several lines of evidence suggest that the suppression or disruption of ACC1 (a cytosolic enzyme that catalyzes the carboxylation of acetyl-CoA to form malonyl-CoA) leads to the reduction of the synthesis and accumulation of hepatic TGs30, suggesting that suppression of ACC1 expression may be due to the reduction of hepatic fat accumulation in Benifuuki-fed mice.
FAS catalyzes the last step in the biosynthetic pathway of fatty acids. Hence, it is believed to be a determinant of the maximal capacity of a tissue (the liver in particular) to synthesize fatty acids by de novo lipogenesis. Increases in FAS levels are attributed to elevation of serum levels of TG and NFEAs as well as liver TG31. Hence, downregulation of FAS expression might lead to a reduction in lipid levels in the liver and serum in Benifuuki-fed mice. SCD1 (which catalyzes the biosynthesis of monounsaturated fatty acids from saturated fatty acids) also has an important role in energy metabolism and regulation of body weight32. Importantly, Benifuuki intake significantly downregulated SCD1 expression. SCD1 plays an essential role in monounsaturated fatty acids for hepatic lipogenesis/TAG synthesis33. Treatment of mice with SCD1 antisense oligonucleotides has been shown to result in a higher metabolic rate, prevention of diet-induced obesity and steatosis34. Therefore, decreased SCD1 expression could explain the reduction in body fat observed in Benifuuki-fed mice. The expression of lipogenic genes such as ACC1, FAS and SCD1 is regulated by SREBP-1c at the transcriptional level35,36. Our data suggested that Benifuuki suppresses the expression of SREBP-1c and results in the reduction of hepatic lipogenic genes (ACC1, FAS and SCD1). Yabukita and Benifuuki were not influenced on genes related to the oxidation of fatty acids (e.g., ACO and MCAD). Taken together, a plausible mechanism for the hypolipidemic activity of Benifuuki may be its downregulation of gene expression associated with lipid synthesis and not with fatty-acid oxidation.
The upregulation of hepatic HMGCS and HMGCR mRNA by HF/HS is consistent with the increased concentration of cholesterol in plasma. Our data showed that green tea suppresses genes associated with cholesterol synthesis (HMGCS, HMGCR) and upregulates CYP7A1 expression in the liver. Yabukita and Benifuuki, however, were ineffective in preventing the HF/HS-induced increase in concentrations of TC in plasma. The effects of green tea on suppression of the genes that regulate cholesterol metabolism were unrelated to TC accumulation in plasma. The reduction of plasma levels of LDL-C/VLDL-C in Benifuuki-fed mice might have been due to suppression of the expression of lipogenesis genes.
Many of the beneficial effects of green tea are considered to be mediated by tea catechin, especially EGCG (the most abundant catechin found in green tea). Several studies have shown that EGCG has anti-obesity effects37,38. There are no significant differences in catechin composition between Benifuuki and Yabukita extracts except for methylated catechins. EGCG3”Me constitutes 6.8% of the total catechins in Benifuuki. It has been reported that if Benifuuki extracts are administered orally to humans, the area under the drug concentration–time curve (AUC) of EGCG3”Me is higher than that of EGCG even though the dose of EGCG is 5.1-times the dose of EGCG3”Me21. Clinical trials have confirmed that EGCG3”Me is absorbed six-times more quickly than EGCG in humans and that it disappears more slowly from the blood than EGCG21. The level of EGCG plus EGCG3”Me in plasma from Benifuuki-administered mice was 5.2 times higher than levels in Yabukita-administered mice. However, the content of EGCG3”Me in Benifuuki was about one-quarter of EGCG's content (Supplementary Fig. 1). It is unlikely that a single substance is responsible for its many beneficial effects because green tea contains many substances. However, with regard to the greater hypolipidemic effect of Benifuuki than Yabukita, EGCG3”Me is probably play a essential role in its bioactivity because large amounts are present in Benifuuki but not in Yabukita. The safety of Benifuuki has been confirmed the result of serum ALT and AST. Furthermore, there are no differences in the parameters for the analyses of blood and urine, pathological examination, or subjective symptoms before and after clinical trials involving long-term treatment with Benifuuki21.
In summary, the present study provides new evidence that tea containing unique methylated catechins such as EGCG3”Me significantly downregulates the hepatic expression of lipid-synthesis genes such SREBP1-c, ACC1, FAS and SCD1. Based on the data and information available, the TG-lowering effect of Benifuuki in plasma and the liver may be mediated (at least in part) via the suppression of lipogenesis, probably because of the high absorption rate and stability of EGCG3”Me in plasma and its effect on lipogenesis genes. However, studies focusing on whether EGCG3”Me has lipid-lowering effects in vivo and in vitro are lacking. Therefore, further studies are needed to better understand the mechanism of Benifuuki and EGCG3”Me.
Methods
Mice and diet
Male C57/BL6 mice (12 weeks) were purchased from Charles River (Kanagawa, Japan). They were maintained in a temperature- and humidity-controlled room with a 12-h-light–dark cycle (light from 8 am to 8 pm). All mice were acclimated for 1 week while being fed an AIN-93G diet. AIN-93G and HF/HS diets were obtained from KBT Oriental (Tokyo, Japan) (Table 3).
Mice were assigned to six groups: (1) a negative control group (control) fed a AIN-93G diet; (2) a positive control group (HF/HS) fed a high-fat/high-sucrose diet; (3) a group (Yabukita 0.2%) fed the HF/HS diet but containing 0.2% Yabukita; (4) a group (Yabukita 1.0%) fed a HF/HS diet containing 1.0% Yabukita; (5) a group (Benifuuki 0.2%) fed a HF/HS containing 0.2% Benifuuki; and (6) a group (Benifuuki 1.0%) fed the HF/HS diet containing 1.0% Benifuuki.
Extracts of Yabukita and Benifuuki were prepared from the leaves of the plants using boiling water followed by freeze-drying. The composition of each extract powder was determined by high-performance liquid chromatography (HPLC) (Table 1). The dietary levels of green tea at 0.2% and 1.0% were equivalent to human intakes of 1.6 cups and 8 cups (2.0 g of green tea/cup) per day, respectively, as estimated based on an energy intake of 8,360 kJ/day. Mice were fed the diets three times a week and were weighed every week. At the end of 6 weeks of feeding, mice were anesthetized under isoflurane vapor after overnight food deprivation. Blood samples were collected into tubes via the retro-orbital sinus. Plasma was collected after centrifugation (2000 × g for 20 min at 4°C) and stored at −80°C. After blood collection, mice were killed by isoflurane overdose. Visceral adipose tissues (peritoneal and epididymal depots) and livers were harvested, rinsed and weighed. From the central lobe of the liver, ~ 0.5 g tissue was excised from each mouse and placed in a plastic tube containing RNALater™ solution (Ambion, Austin, TX, USA). Liver tissue in RNALater was maintained at 4°C for 24 h and then stored at −80°C. This experiment was carried out according to the guidelines for animal experiments at the Faculty of Agriculture, Kyushu University. The study protocol was approved by the Animal Care and Use Committee of Kyushu University, Fukuoka, Japan. The approval number for the animal experiment is A22-146.
Measurement of the amount of catechins and caffeine in tea extracts
Tea samples were prepared for HPLC analyses. Briefly, samples were immersed in 50% ethanol containing 1% H3PO4, disrupted using ultrasonic apparatus for 30 min at 20°C and then filtered through a membrane filter (pore size, 0.45 μm). HPLC was undertaken using a Shimadzu LC-10A pump coupled to an ultraviolet detector (SPD-M10Avp; Shimadzu, Kyoto, Japan) and a reverse-phase Wakopak Navi C18-5 column (4.6 mm i.d. × 150 mm; granule diameter, 5 μm; Wako Pure Chemical Industries, Kyoto, Japan). Elution consisted of a 2−45-min linear gradient from 0 to 80% of solvent B and solvent A. Solvent A consisted of distilled water/phosphoric acid/acetonitrile (400:10:1 v/v) and solvent B consisted of methanol/solvent A (1:2 v/v). Samples were eluted at 1 mL/min at 40°C. The detection wavelength was 272 nm. The amounts of catechins and caffeine in tea extracts were measured by comparing the peak area of each catechin in the tea extract with that of a standard preparation that contained a fixed quantity of caffeine, EC, C, EGCG, gallocatechin gallate (GCG), ECG, catechin gallate (CG), EGCG3”Me and gallocatechin-3-O-(3-O-methyl) gallate (GCG3″Me).
Biochemical analyses of plasma and liver samples
Plasma levels of triglyceride (TG), non-esterified fatty acids (NEFAs), aspartate aminotransferase (AST), alanine transaminase (ALT), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C) and very low-density lipoprotein-cholesterol (VLDL-C) were measured using the TG E-test, the non-esterified fatty acid (NEFA) C-test and the transaminase CII-test (each from Wako), HDL-C and LDL-C/VLDL-C Quantification Kit (BioVision, Milpitas, CA, USA), respectively. The liver TG content were measured using the TG E-test (from Wako), respectively, after the extraction of hepatic lipids with chloroform-methanol (2:1). All kits were used in accordance with manufacturer instructions.
RNA isolation and DNA chip analyses
Total RNA was extracted from liver samples using the RNeasy™ Mini Kit (Qiagen, Valencia, CA, USA). All total RNA samples were analyzed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). Biotinylated antisense RNA was synthesized and amplified from total RNA (1 μg) using the MessageAmpII Biotin Enhanced Amplification kit (Applied Biosystems, Foster City, CA, USA) according to manufacturer protocols. After aRNA purification, biotinylated aRNA (5 μg) was fragmented using 10 × fragmentation reagents (Applied Biosystems) by heating at 70°C for 7.5 min. Hybridization was carried out with DNA chips in 150 μL hybridization buffer (0.12 M Tris HCl/0.12 M NaCl/0.05% Tween-20 and 5 μg fragmented biotinylated aRNA) at 65°C overnight. After hybridization, DNA chips were washed twice in 0.12 M Tris HCl/0.12 M NaCl/0.05% Tween-20 at 65°C for 20 min, followed by washing in 0.12 M Tris HCl/0.12 M NaCl for 10 min. Then hybridized aRNA was labeled with 2 μg/mL streptavidin-Cy5 (GE Healthcare, Little Chalfont, UK) in 0.12 M Tris HCl/0.12 M NaCl for 30 min at room temperature. After fluorescent labeling, DNA chips were washed four times in 0.12 M Tris HCl/0.12 M NaCl/0.05% Tween-20 at room temperature for 5-min each. DNA chips were scanned at multiple exposure times (0.1–40 s) using a DNA Chip Reader (Yokogawa Electric, Tokyo, Japan) with multi-beam excitation technology. The intensity values with the best exposure condition for each spot were selected. The median value of background spots was subtracted from the intensity value in each gene and thereafter the value was normalized to the expression of an endogenous control (acidic ribosomal phosphoprotein, beta-actin and glyceraldehyde-3-phosphate dehydrogenase.
Real-time quantitative polymerase chain reaction (PCR)
cDNA was synthesized from total RNA (1 μg) using the PrimeScript RT reagent Kit (Takara Bio, Tokyo, Japan). Gene expression was analyzed by real-time quantitative PCR using the Sybr green procedure and a Thermal Cycler Dice Real Time System (Takara Bio). Primer sequences are given in Supplementary Table S2. Expression of mRNA values was normalized relative to glyceraldehyde-3-phosphate dehydrogenase as an internal control.
Statistical analyses
All statistical analyses were performed using one-way ANOVA with Tukey's post hoc test using Kyplot software. Data are the means ± SEM. P < 0.05 was considered significan.
References
Després, J. P. & Lemieux, I. Abdominal obesity and metabolic syndrome. Nature. 444, 881–887 (2006).
Fernandez, M. L. & Webb, D. The LDL to HDL cholesterol ratio as a valuable tool to evaluate coronary heart disease risk. J. Am. Coll. Nutr. 27, 1–5 (2008).
Yang, K. et al. Dietary induction of colonic tumors in a mouse model of sporadic colon cancer. Cancer Res. 68, 7803–7810 (2008).
Erdelyi, I. et al. Western-style diets induce oxidative stress and dysregulate immune responses in the colon in a mouse model of sporadic colon cancer. J Nutr. 139, 2072–2078 (2009).
Yung, L. M. et al. Tea polyphenols benefit vascular function. Inflammopharmacology. 16, 230–234 (2008).
Bors, W. & Saran, M. Radical scavenging by flavonoid antioxidants. Free Radic Res Commun. 2, 289–294 (1987).
Murase, T., Nagasawa, A., Suzuki, J., Hase, T. & Tokimitsu, I. Beneficial effects of tea catechins on diet-induced obesity: stimulation of lipid catabolism in the liver. Int J Obes Relat Metab Disord. 26, 1459–1464 (2002).
Fujimura, Y., Tachibana, H. & Yamada, K. Tea catechin suppresses the expression of the high-affinity IgE receptor Fc epsilon RI in human basophilic KU812 cells. J Agric Food Chem. 50, 5729–5734 (2002).
Tachibana, H., Koga, K., Fujimura, Y. & Yamada, K. A receptor for green tea polyphenol EGCG. Nat Struct Mol Biol. 11, 380–381 (2004).
Tsukamoto, S. et al. Green tea polyphenol EGCG induces lipid-raft clustering and apoptotic cell death by activating protein kinase Cδ and acid sphingomyelinase through a 67 kDa laminin receptor in multiple myeloma cells. Biochem J. 443, 525–534 (2012).
Hong, B. E., Fujimura, Y., Yamada, K. & Tachibana, H. TLR4 signaling inhibitory pathway induced by green tea polyphenol epigallocatechin-3-gallate through 67-kDa laminin receptor. J Immunol. 185, 33–45 (2010).
Umeda, D., Yano, S., Yamada, K. & Tachibana, H. Green tea polyphenol epigallocatechin-3-gallate signaling pathway through 67-kDa laminin receptor. J Biol Chem. 283, 3050–3058 (2008).
Byun, E. H., Omura, T., Yamada, K. & Tachibana, H. Green tea polyphenol epigallocatechin-3-gallate inhibits TLR2 signaling induced by peptidoglycan through the polyphenol sensing molecule 67-kDa laminin receptor. FEBS Lett. 585, 814–820 (2011).
Ueda, M. et al. Tea catechins modulate the glucose transport system in 3T3-L1 adipocytes. Food Funct. 1, 167–173 (2010).
Ueda, M. et al. Epigallocatechin gallate promotes GLUT4 translocation in skeletal muscle. Biochem Biophys Res Commun. 377, 286–290 (2008).
Lin, Y. L. & Lin, J. K. (−)-Epigallocatechin-3-gallate blocks the induction of nitric oxide synthase by down-regulating lipopolysaccharide-induced activity of transcription factor nuclear factor-kappaB. Mol Pharmacol. 52, 465–472 (1997).
Westerterp-Plantenga, M. S., Lejeune, M. P. & Kovacs, E. M. Body weight loss and weight maintenance in relation to habitual caffeine intake and green tea supplementation. Obes Res. 13, 1195–1204 (2005).
Nagao, T. et al. Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde-modified LDL in men. Am J Clin Nutr. 81, 122–1229 (2005).
Moon, H. S., Lee, H. G., Choi, Y. J., Kim, T. G. & Cho, C. S. Proposed mechanisms of (-)- epigallocatechin-3-gallate for anti-obesity. Chem Biol Interact. 167, 85–98 (2007).
Chen, Y. K. et al. Effects of green tea polyphenol (-)-epigallocatechin-3-gallate on newly developed high-fat/Western-style diet-induced obesity and metabolic syndrome in mice. J. Agric. Food Chem. 59, 11862–71 (2011).
Maeda-Yamamoto, M., Ema, K. & Shibuichi, I. In vitro and in vivo anti-allergic effects of ‘Benifuuki’ green tea containing O-methylated catechin and ginger extract enhancement. Cytotechnology. 55, 135–142 (2007).
Maeda-Yamamoto, M. et al. The efficacy of early treatment of seasonal allergic rhinitis with benifuuki green tea containing O-methylated catechin before pollen exposure: an open randomized study. Allergol Int. 58, 437–444 (2009).
Maeda-Yamamoto, M. et al. Effect of green tea powder (Camellia sinensis L. cv. Benifuuki) particle size on O-methylated EGCG absorption in rats; The Kakegawa Study. Cytotechnology. 63, 171–179 (2011).
Yang, Z. H., Miyahara, H., Takeo, J. & Katayama, M. Diet high in fat and sucrose induces rapid onset of obesity-related metabolic syndrome partly through rapid response of genes involved in lipogenesis, insulin signalling and inflammation in mice. Diabetol Metab Syndr. 4, 32 (2012).
Kajikawa, S., Harada, T., Kawashima, A., Imada, K. & Mizuguchi, K. Highly purified eicosapentaenoic acid prevents the progression of hepatic steatosis by repressing monounsaturated fatty acid synthesis in high-fat/high-sucrose diet-fed mice. Prostaglandins Leukot Essent Fatty Acids. 80, 229–238 (2009).
Sato, A. et al. Antiobesity effect of eicosapentaenoic acid in high-fat/high-sucrose diet-induced obesity: importance of hepatic lipogenesis. Diabetes. 59, 2495–2504 (2010).
Rutledge, A. C. & Adeli, K. Fructose and the metabolic syndrome: pathophysiology and molecular mechanisms. Nutr Rev. 65, S13–23 (2007).
Miyazaki, M. et al. Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and -independent mechanisms. J Biol Chem. 279, 25164–25171 (2004).
Matsuzaka, T. et al. Insulin-independent induction of sterol regulatory element-binding protein-1c expression in the livers of streptozotocin-treated mice. Diabetes. 53, 560–569 (2004).
Mao, J. et al. Liver-specific deletion of acetyl-CoA carboxylase 1 reduces hepatic triglyceride accumulation without affecting glucose homeostasis. Proc Natl Acad Sci USA. 103, 8552–8557 (2006).
Ai, Z. L. et al. The role of hepatic liver X receptor α- and sterol regulatory element binding protein-1c-mediated lipid disorder in the pathogenesis of non-alcoholic steatohepatitis in rats. J Int Med Res. 39, 1219–1229 (2011).
Dobrzyn, A. & Ntambi, J. M. The role of stearoyl-CoA desaturase in body weight regulation. Trends Cardiovasc Med. 14, 77–81 (2004).
Ntambi, J. M. et al. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci USA. 99, 11482–11486 (2002).
Jiang, G. et al. Prevention of obesity in mice by antisense oligonucleotide inhibitors of stearoyl-CoA desaturase-1. J Clin Invest. 115, 1030–1038 (2005).
Ferré, P. & Foufelle, F. SREBP-1c transcription factor and lipid homeostasis: clinical perspective. Horm Res. 68, 72–82 (2007).
Shimano, H. SREBPs: physiology and pathophysiology of the SREBP family. FEBS J. 276, 616–621 (2009).
Chen, Y. K. et al. Effects of Green Tea Polyphenol (−)-Epigallocatechin-3-gallate on Newly Developed High-Fat/Western-Style Diet-Induced Obesity and Metabolic Syndrome in Mice. J Agric Food Chem. 59, 11862–11871 (2011).
Grove, K. A., Sae-Tan, S., Kennett, M. J. & Lambert, J. D. (-)-Epigallocatechin-3-gallate Inhibits Pancreatic Lipase and Reduces Body Weight Gain in High Fat-Fed Obese Mice. Obesity. 20, 2311–2313 (2012).
Author information
Authors and Affiliations
Contributions
T.S. M.K. T.H. K.Y. and H.T. designed the research. T.S. M.K. Y.K. S.Y. S.T. M.S. K.N. T.H. Y.T. S.Y. Y.H. K.N. M.M. Y.S. W.Y. K.J. and H.T. conducted the research. S.T. T.H. Y.T. and H.T. analyzed data and undertook statistical analyses. T.S. and H.T. wrote the manuscript. H.T. had primary responsibility for the final content. All authors approved the final manuscript for submission.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Figure 1, Supplemental table 1,Supplemental table 2
Rights and permissions
This work is licensed under a Creative Commons Attribution 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by/3.0/
About this article
Cite this article
Suzuki, T., Kumazoe, M., Kim, Y. et al. Green Tea Extract Containing a Highly Absorbent Catechin Prevents Diet-Induced Lipid Metabolism Disorder. Sci Rep 3, 2749 (2013). https://doi.org/10.1038/srep02749
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep02749
This article is cited by
-
Bioactivity-boosting strategy based on combination of anti-allergic O-methylated catechin with a Citrus flavanone, hesperetin
Journal of Natural Medicines (2023)
-
Methylated (−)-epigallocatechin 3-O-gallate potentiates the effect of split vaccine accompanied with upregulation of Toll-like receptor 5
Scientific Reports (2021)
-
Glucosyl-hesperidin enhances the cyclic guanosine monophosphate-inducing effect of a green tea polyphenol EGCG
Journal of Natural Medicines (2021)
-
Dietary supplement of Yunkang 10 green tea and treadmill exercise ameliorate high fat diet induced metabolic syndrome of C57BL/6 J mice
Nutrition & Metabolism (2020)
-
Postprandial glycaemia-lowering effect of a green tea cultivar Sunrouge and cultivar-specific metabolic profiling for determining bioactivity-related ingredients
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.