Abstract
Polyreactive antibodies are a major component of the natural antibody repertoire and are capable of binding a variety of structurally unrelated antigens. Many of the properties attributed to natural antibodies, in fact, are turning out to be due to polyreactive antibodies. In humans, each day, billions of cells undergo apoptosis. In the present experiments, we show by ImageStream technology that although polyreactive antibodies do not bind to live T cells they bind to both the plasma membrane and cytoplasm of late apoptotic cells, fix complement, generate the anaphylatoxin C5a and increase by as much as 5 fold complement-mediated phagocytosis by macrophages. Of particular importance, T cells undergoing apoptosis following infection with HIV also bind polyreactive antibodies and are phagocytosed. We conclude that the polyreactive antibodies in the natural antibody repertoire contribute in a major way to the clearance of cells made apoptotic by a variety of natural and infectious processes.
Similar content being viewed by others
Introduction
Natural antibodies have been known for well over 100 years, but have remained an enigma because they are found in the absence of known antigenic exposure and are present in newborns and germ-free animals1. The function of these antibodies has been widely debated, but they are now generally thought to serve as a first line of defense against foreign invaders and are considered part of the innate immune system2,3,4,5,6,7,8,9,10,11,12. Adding to the complexity of natural antibodies, however, is the fact that many of these antibodies react with normal host proteins suggesting that some may be autoantibodies or the precursors of autoantibodies13,14.
Since normal sera contain millions of different natural antibody molecules, all in small quantities, it has been difficult to characterize these antibodies15. However, with the advent of hybridoma technology it became possible to prepare large quantities of individual natural antibody molecules. Analysis of monoclonal antibodies from normal individuals showed that, in fact, many were polyreactive, that is they could bind to a variety of structurally unrelated self and non-self antigens2,3,4,5,7,16. In contrast to monoreactive antibodies, polyreactive antibodies have a low binding affinity for antigens and many have a germ-line or near germ-line configuration. The antigen-binding pocket of these antibodies are thought to be more flexible than monoreactive antibodies and thereby can accommodate different antigenic configurations5. Further studies on monoclonal polyreactive antibodies showed that they are a major component of the natural antibody repertoire and represent about 50% of the B cells in the cord blood of newborns and15% to 20% of the B cells in the peripheral circulation17,18.
The biological function of polyreactive antibodies, however, has not been fully evaluated. Recently, using a panel of monoclonal polyreactive antibodies we showed that polyreactive antibodies could bind to both Gram-negative and Gram-positive bacteria and that in the presence of complement could inhibit bacterial growth11. In addition, those studies showed that polyreactive antibody-enriched, but not polyreactive antibody-depleted, IgM prepared from normal human sera displayed antibacterial activity similar to that of monoclonal polyreactive antibodies. Thus, these studies support the argument that the broad antibacterial activity of the natural antibody repertoire is in large part due to the presence of polyreactive antibodies.
Polyreactive antibodies also may contribute to other functions of the natural antibody repertoire. In humans, each day, billions of cells undergo apoptosis19. Numerous studies have shown that natural antibodies bind to apoptotic cells and enhance their phagocytosis by macrophages20,21,22,23,24. The role of polyreactive antibodies in this process, however, has not been clearly defined25,26. The present experiments were initiated to test the hypothesis that polyreactive antibodies in the natural antibody repertoire bind to antigens on the surface and within the cytoplasm of cells made apoptotic by UV light or HIV infection and are an important contributor to the phagocytosis of damaged cells.
Results
Polyreactive antibodies bind to apoptotic T cells
Human T lymphocytes were exposed to UV light for up to 21 minutes (Fig. 1a) and the percentage of apoptotic cells was determined by the binding of Annexin V and the uptake of 7AAD. At time zero, 12.8% of the cells exhibited evidence of apoptosis. This increased to 70% at 6 minutes and to 98% at 21 minutes. Fig. 1b shows that the binding of polyreactive antibody 2E4 increased from 11.5% prior to UV to 92% at 21 minutes post-UV exposure, indicating a strong correlation of polyreactive antibody binding with apoptosis. In contrast to polyreactive antibody 2E4, monoreactive antibody 8512 showed essentially no binding to the apoptotic cells at any of the times examined.
Polyreactive antibody 2E4 binds to UV-induced apoptotic T cells.
Human T cells were incubated with polyreactive 2E4 or monoreactive 8512 after being exposed to UV light for different lengths of time. (a) Apoptosis as evaluated by the percentage of cells binding Annexin V and 7AAD. (b) Polyreactive 2E4, but not monoreactive 8512, binds to apoptotic cells. Data are representative of three independent experiments with T cells from three different donors.
To further define the binding of polyreactive antibodies to apoptotic cells, UV-exposed human T cells were incubated with monoreactive or polyreactive antibodies and gated into three populations (Fig. 2a): live cells; early apoptotic cells (binding of Annexin V); and late apoptotic cells (binding of both Annexin V and the uptake of PI). Neither the monoreactive nor polyreactive antibodies bound to live cells. Monoreactive antibody 8512 also failed to bind to any of the early apoptotic cells and polyreactive antibodies 2E4, ZH-6 and ZH-20 bound only to a small percentage (3.5% to 18.1%) of the early apoptotic cells. In marked contrast, polyreactive antibodies 2E4, ZH-6 and ZH-20 bound to a high percentage of the late apoptotic cells (39.7% to 87.1%), whereas monoreactive antibody 8512 bound to only 1.7% of the late apoptotic cells. Two other monoreactive antibodies (2507 and 8018) yielded results very similar to that observed with monoreactive 8512 (data not shown).
Polyreactive antibodies bind primarily to late apoptotic cells and to multiple sites on and within apoptotic T cells.
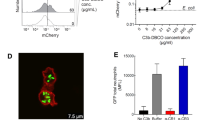
(a) Human T cells exposed to UV light were sorted into live (Annexin V−/PI−), early (Annexin V+/PI−) and late (Annexin V+/PI+) apoptotic populations. The binding profile of monoreactive 8512 and polyreactive 2E4, ZH-6 and ZH-20 antibodies showed that the polyreactive antibodies bound primarily to late apoptotic cells. (b) UV-induced late apoptotic human T cells were incubated with polyreactive 2E4 and then stained with FITC-labeled antibody to CD3 (green) to identify the plasma membrane and Draq 5 (blue) to identify the nucleus. PE-labeled anti-immunoglobulin (red) was used to co-localize polyreactive 2E4 with these structures based upon bright field similarity analysis by ImageStream. 2E4/Draq 5, 2E4/CD3 and the 2E4/Draq 5/CD3 co-localization was quantified based on the fraction of each population present in a total of 10,000 cell images obtained from each of the samples collected. Representative images show co-localization (merge) of 2E4 with nucleus (Draq 5: row 1); 2E4 with plasma membrane (CD3: row 2); and 2E4 with cytoplasm, nucleus and plasma membrane (Draq 5 and CD3: row 3). Controls show staining in the absence of 2E4 (row 4). Experiments (panels a and b) were repeated at least three times and each time with T cells from different donors.
Determination of cellular–binding sites of polyreactive antibodies
Human peripheral blood T cells were made apoptotic by exposure to UV light and then incubated with polyreactive 2E4 and stained with anti-CD3-FITC to locate the cell membrane, Draq5 to locate the nucleus and PE-labeled anti-IgM to locate the binding sites of polyreactive 2E4. Quantification by ImageStream (Figure 2b) showed that polyreactive 2E4 colocalized with just nuclei in 16.5 ± 11.9% of the cells, with just the plasma membrane in 4.5 ± 0.7% of the cells and with the cytoplasm, nucleus and plasma membrane in 67.5 ± 13.4% of the cells. Pictorial analysis by ImageStream shows representative images of the binding of polyreactive 2E4 to: nucleus (row 1); plasma membrane (row 2); and plasma membrane, nucleus and cytoplasm (row 3). Staining of just the membrane and nucleus in the absence of 2E4 is shown in row 4.
Apoptotic cells exposed to polyreactive antibodies fix complement and generate the anaphylatoxin C5a
Human peripheral T cells were made apoptotic by exposure to UV light for 6 minutes. The cells then were incubated with polyreactive antibody 2E4 or monoreactive antibody 8512 in the presence of human complement. As seen in Fig. 3a, complement did not bind to live cells treated with either antibody, but showed substantial binding to apoptotic cells that had been treated with polyreactive antibody 2E4 (57.1%). In contrast, there was comparatively little binding to apoptotic cells treated with PBS or monoreactive antibody 8512 (13.4%). Fig. 3b shows that the binding of polyreactive 2E4 to apoptotic cells not only fixed complement, but also generated the anaphylatoxin C5a, an important chemotaxis factor.
Binding of polyreactive antibodies to human apoptotic T cells fixes complement, generates C5a and enhances phagocytosis by macrophages.
(a) Apoptotic, but not live, T cells fix complement in the presence of polyreactive 2E4, but not monoreactive 8512. Complement binding was determined by antibody to C3. (b) Apoptotic T cells treated with polyreactive 2E4, but not monoreactive 8512, fix complement and generate the anaphylatoxin C5a as determined by mean fluorescence intensity using BD Cytometric Bead Array. (c) In the presence of complement, there is a 3.0 to 5.0 fold increase in the phagocytosis of apoptotic T cells treated with polyreactive antibodies 2E4, ZH-6, ZH-20 and ZH-14 as compared to cells treated with monoreactive antibody 8512 or PBS. In the absence of complement, there is no increase in phagocytosis over background activity. FACS analysis shows gated PKH26 macrophages. PKH67 single positive T cells were excluded in the analysis.
Polyreactive antibody-binding enhances complement-mediated phagocytosis
To determine whether the binding of polyreactive antibodies to apoptotic T cells, in the presence of human complement, enhances phagocytosis, human apoptotic T cells (stained green with PKH67) were incubated with Raw 264.7 macrophages (stained red with PKH26). Phagocytosis was evaluated by FACS analysis and by ImageStream. As seen in Fig. 3c, in the absence of complement, essentially none of the T cells that had been exposed to polyreactive antibodies were phagocytosed by macrophages (~5.3 to 7.7%) as compared to cells exposed to monoreactive 8512 (5.6%) or PBS (6.42%). In the presence of complement there was essentially no increase in the phagocytosis of apoptotic T cells exposed to monoreactive 8512 (6.7%) as compared to PBS (4.8%), but a 3.0 to 5.0 fold increase in the phagocytosis of T cells exposed to polyreactive antibodies 2E4, ZH-6, ZH-20 and ZH-14 (14.7% to 25.4%).
In vivo phagocytosis of apoptotic murine thymic T cells treated with polyreactive antibody and complement
As an in vivo correlate, IgM-deficient mice were injected intraperitoneally with PKH26 solution to stain macrophages red. Murine apoptotic thymic T cells, stained green with PKH67 and treated with either polyreactive 2E4 and complement or monoreactive 8512 and complement, then were injected into the peritoneal cavity. Approximately 90 minutes later peritoneal macrophages were isolated and analyzed pictorially by ImageStream for double staining as evidence of phagocytosis. As seen in Fig. 4a, 58% of the gated macrophages phagocytosed thymic T cells that had been incubated with polyreactive antibody 2E4 and human complement (PKH26+/PKH67+). In contrast only 33% of the gated macrophages phagocytosed thymic T cells that had been incubated with monoreactive antibody 8512 and complement (Figure 4b). Representative images of phagocytosis from Figure 4a (co-localization of thymic T cells with macrophages) are shown in panels 4d–f. Delta centroid XY analysis (Figure 4c) by ImageStream revealed that 76.1 ± 4.1% of the double positive cells were fully phagocytosed; 4.6 ± 1.5% were partially phagocytosed; and 1.4 ± 0.8% simply showed macrophage/T cell adherence. Delta centroid XY analysis of cells treated with monoreactive antibody 8512 and that were PKH26+/PKH67+ revealed images similar to those in panels d–f (not shown).
In vivo phagocytosis of apoptotic thymic T cells treated with polyreactive 2E4 plus complement or monoreactive 8512 plus complement.
IgM-deficient mice were injected with PKH26 solution to stain peritoneal macrophages and 24 hours later injected with apoptotic thymic T cells that had been stained with PKH67. Analysis by ImageStream of gated peritoneal macrophages showed (a) that nearly twice as many of the macrophages phagocytosed thymic T cells (PKH26+/PKH67+) that had been exposed to polyreactive 2E4, as compared to (b) thymic T cells that had been exposed to monoreactive 8512. Gating was based upon the negative control, i.e., PKH26-stained macrophages without exposure to PKH67-stained thymic cells. (c) PKH26+/PKH67+ cells from Figure 4a then were divided into fully phagocytosed, partially phagocytosed and adherent cells based upon Delta Centroid XY analysis. (d–f) Representative images taken by ImageStream showing phagocytosis (merge) of apoptotic thymic T cells (green) by macrophages (red): (d) fully phagocytosed; (e) partially phagocytosed; and (f) macrophage/thymic T cell adherence. Data are representative of at least three different experiments.
Polyreactive antibodies bind to HIV-induced apoptotic cells
Human peripheral CD4+ T lymphocytes were infected with HIV and 8 days later incubated with monoreactive or polyreactive antibodies and stained for Annexin V and anti-CD4 binding. Infected CD4+/Annexin V+ apoptotic cells (Fig. 5a) and uninfected control CD4+/Annexin V− (Fig. 5b) cells then were evaluated for the expression of gp120 and the binding of monoreactive or polyreactive antibody. As seen in Fig. 5a, polyreactive antibodies (2E4, ZH-6 and ZH-20) bound to the gp120+ apoptotic cells, but not to the gp120− non-apoptotic control cells (Fig. 5b).The monoreactive antibody 8512 bound to neither the uninfected control cells (Fig. 5b) nor the HIV-infected apoptotic cells (Fig. 5a).
Polyreactive antibodies bind to HIV-induced apoptotic cells.
HIV-infected CD4+ T cells were gated for apoptosis as determined by Annexin V binding. (a) Polyreactive antibodies 2E4, ZH-6 and ZH-20, but not monoreactive antibody 8512, bound to the HIV-infected apoptotic T cells. (b) Neither monoreactive nor polyreactive antibodies bound to the non-infected cells. (c) Human T cells gated by ImageStream into Annexin V−/gp120+, Annexin V+/gp120+ and double negative cells. (d) Representative images showing that Annexin V+/gp120+ apoptotic cells bind polyreactive 2E4 antibody, but not (e) monoreactive 8512 antibody.
To evaluate pictorially the binding of polyreactive 2E4 to HIV-induced apoptotic cells, ImageStream was used. Cells were gated into: Annexin V−/gp120+; Annexin V+/gp120+; and Annexin V−/gp120− (Fig. 5c). Figs. 5d and 5e show, respectively, three representative Annexin V+/gp120+ apoptotic cells to which polyreactive antibody 2E4 bound (merge) and three representative Annexin V+/gp120+ apoptotic cells to which monoreactive antibody 8512 did not bind. Annexin V−/gp120− cells and Annexin V−/gp120+ cells showed little or no binding of polyreactive antibodies (data not shown).
The binding of polyreactive antibody to HIV-induced apoptotic cells enhances phagocytosis
Human T cells stained with PKH67 were infected with HIV and 8 days later the cells were harvested and incubated with polyreactive 2E4 or monoreactive 8512 in the presence of complement. The T cells then were incubated with PKH26 stained macrophages and phagocytosis was evaluated by ImageStream. Whereas only 11.2% of the gated macrophages phagocytosed HIV-infected T cells (PKH26+/PKH67+/gp120+) that had been incubated with monoreactive antibody 8512 plus complement (Figure 6b), 20% of the gated macrophages phagocytosed HIV-infected T cells that had been incubated with polyreactive 2E4 plus complement (Figure 6a). Fig. 6d–f show representative images of polyreactive antibody-mediated phagocytosis of gp120+ T cells (green/APC+) by macrophages (red) (merge). Delta centroid XY analysis (Figure 6c) revealed that 41.2 ± 8% of the triple positive cells were fully phagocytosed; 35.1 ± 7.1% were partially phagocytosed; and 12.4 ± 6.1% simply showed macrophage/T cell adherence. Delta centroid XY analysis of cells treated with monoreactive antibody 8512 and that were PKH26+/PKH67+/gp120+ revealed images similar to those in panels c–f (not shown).
Phagocytosis of HIV-induced apoptotic T cells by macrophages as determined by ImageStream.
Analysis by ImageStream of macrophages showing (a) that nearly twice as many of the HIV-infected (gp120+)T cells that had been exposed to polyreactive 2E4 and complement, as compared to (b) monoreactive 8512 and complement, were phagocytosed by macrophages. Macrophages were gated based upon PKH26 positivity and the PKH26+/PKH67+/gp120+ population was analyzed. (c) Polyreactive 2E4-treated HIV infected T cells (PKH67+/gp120+) phagocytosed by macrophages (PKH26+) were analyzed based upon Delta Centroid XY analysis. (d–f) Representative images showing phagocytosis (merge) of HIV infected T cells (green and APC+) by macrophages (red): (d) fully phagocytosed; (e) partially phagocytosed; and (f) macrophage/T cell adherence. Data are representative of at least three different experiments.
Polyeactive antibodies isolated from human sera bind to apoptotic cells
Polyreactive antibodies in human sera were isolated and enriched by passage of human serum IgM through a heparin column as described in Materials and Methods. The polyreactive-enriched and reduced IgM were concentrated and tested for binding to live and UV-induced early and late apoptotic T cells. As seen in Fig. 7, neither the polyreactive-enriched nor reduced IgM from sera bound to live cells and only minimally to early apoptotic cells. In contrast, the polyreactive-enriched IgM bound to 53% of the late apoptotic cell, whereas the polyreactive-reduced IgM bound to only16% of the late apoptotic cells. These findings show directly that a substantial portion of the antibodies in the natural antibody repertoire that bind to apoptotic cells are polyreactive antibodies.
Polyreactive–enriched, but not polyreactive-reduced, IgM from human serum binds to UV-induced apoptotic cells.
(a) Human T cells exposed to UV light were sorted into live (Annexin V−/7AAD−), early (Annexin V+/7AAD−) and late (Annexin V+/7AAD+) apoptotic populations. (b) The binding profile of polyreactive-enriched IgM and polyreactive-reduced IgM to apoptotic cells.
Discussion
Under normal physiologic conditions, in humans, it is estimated that about ten billion cells a day undergo apoptosis19. Apoptosis is critical in a variety of biological processes ranging from the pruning and remodeling of organs during development to the removal of damaged cells in various disease states23,27,28,29,30,31,32. Grabar was one of the first to suggest that natural antibodies might play a “house-keeping” role in the removal of damaged cells from the body to maintain homeostasis33,34. In the present study, we showed that polyreactive antibodies, which are a major component in the natural antibody repertoire, can mediate this removal of damaged cells. We found that within minutes after exposure of human T cells to UV light, 97% of the cells became apoptotic as demonstrated by the binding of Annexin V and the uptake of 7AAD or PI. Incubation of these cells with monoclonal antibodies revealed that up to 90% of the apoptotic cells bound polyreactive antibodies, whereas less that 2% bound monoreactive antibodies.
Somewhat surprising, the four monoclonal polyreactive antibodies that we tested failed to bind to the surface of viable T cells despite the fact that polyreactive antibodies can bind to a variety of large and small (peptides) molecules. In fact, recently it was found that when polyreactive antibody 2E4 was screened with a 10,000 random peptide array, it bound to hundreds of totally unrelated peptides35. Although at the present time we have no explanation for the failure of polyreactive antibodies to bind to the surface of viable T cells one possibility is that there are protective molecules on the surface of these cells which inhibit the binding of polyreactive antibodies. From an evolutionary point of view, the binding of polyreactive antibodies to the surface of viable T cells would be harmful to the host if, in the presence of complement, this resulted in cell lysis. The failure of polyreactive antibodies to bind to the surface of viable cells is not limited to human peripheral T cells, but also was observed with viable MIN-6, AtT-20, NCI-H345, NCI-H82 cells and mouse T cells (not shown).
In regard to the apoptotic cells, ImageStream technology made it possible to determine and quantitate the sites on and within the cells to which the polyreactive antibodies bound and to quantitate phagocytosis by macrophages. Our studies showed that in late apoptosis, polyreactive antibodies readily bound to newly exposed antigens on the cell surface as well as to antigens within the cytoplasm and nucleus. This is consistent with earlier studies, using formaldehyde-fixed cells, which showed by immunofluorescence microscopy the binding of polyreactive antibodies to a variety of antigens within the cytoplasm of cells from different organs and cell types2,3,16. Thus, late apoptotic cells are permeable and polyreactive antibodies can enter the cytoplasm and bind to antigens unrelated to conventional surface apoptotic markers. Taken together, we conclude from these studies that the natural antibody repertoire contains both monoreactive antibodies, (e.g., T15)36, which bind to apoptotic-related antigens (e.g., phosphorylcholine)23 and now polyreactive antibodies (e.g., 2E4) which bind to both apoptotic-related (Supplement Fig. 1) and non-apoptotic-related antigens15.
As previously shown with sera containing natural antibodies20,21,24,37, the present study shows that the binding of monoclonal polyreactive antibodies to late apoptotic cells leads to the fixation of complement and the generation of the anaphylatoxin C5a which has chemotactic properties. Moreover, the apoptotic cells to which polyreactive antibodies and complement bind are up to 5 times more likely to be phagocytosed by macrophages than apoptotic cells treated with monoreactive antibodies in either the presence or absence of complement. This contrasts with the findings of Fu, et.al.25 who argue that phagocytosis of apoptotic cells by polyreactive antibody can occur in a complement-independent fashion. In their experiments, carried out in the absence of complement, phagocytosis was increased by only a relatively small amount from 40% in cells not treated with polyreactive antibody to about 55% in cells treated with polyreacitve antibody. Based on our findings we conclude that complement is critical to the optimal functioning of polyreactive antibodies.
Of particular interest, in our experiment cells made apoptotic by infection with HIV also bound polyreactive antibodies and complement. As in the case of UV-induced apoptotic cells, HIV-induced apoptotic cells treated with polyreactive antibody and complement also are more likely to be phagocytosed by macrophages than HIV-infected cells treated with irrelevant monoreactive antibodies and complement. Since a variety of viral infections can lead to apoptosis and there are millions of different polyreactive antibody molecules in the natural antibody repertoire, the current study argues that polyreactive antibodies are an important component in the host's innate defense which involves the phagocytosis and clearance of virus-infected apoptotic cells.
Support that the findings described here are not just an in vitro phenomenon comes from the experiments in which UV-induced apoptotic thymic T cells, which had been treated with polyreactive antibody and complement, were injected intraperitoneally into IgM-deficient mice (Fig. 4). This experiment showed that peritoneal macrophages were considerably more efficient in phagocytosing the polyreactive antibody-treated apoptotic cells than the monoreactive antibody-treated apoptotic cells. These findings also point to the possibility that polyreactive antibodies, which are predominately IgM but also may be IgG, in IV-Ig preparations may be responsible for some of the therapeutic effects of IV-Ig38,39,40,41. Conversely, it is interesting to speculate that a deficiency in polyreactive antibodies may contribute to the pathogenesis of some immune disorders such as systemic lupus erythematosus, which are thought to be triggered by impaired clearance of apoptotic cells21,28,29.
Because of their low binding affinity, especially in liquid phase, polyreactive antibodies have not been considered immunologically important. However, based on the fact that polyreactive antibodies readily bind to antigens in solid phase reactions, such as to bacteria, viruses and now apoptotic cells and also can fix complement, the importance of these antibodies in both natural defense and in the clearance of apoptotic cells needs to be reconsidered.
Methods
Mice, primary cells and cell lines
Mice
Three- to six-week-old C57BL mice were bred at the NIH animal facility (Bethesda, MD). Six to 8 week-old C57BL/10-Igh-6tmlCgn (IgM−/−, μMT) mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). All experiments were carried out in compliance with institutional guidelines and approved by the NIDCR ACUC (Bethesda, MD). All mice were housed under specific pathogen-free conditions.
Murine thymus cells
Thymus tissue from C57BL mice was gently minced in cold PBS containing 2 mM EDTA and 2% BSA and filtered through a 70 μM cell strainer (BD Falcon), centrifuged and re-suspended in cold PBS.
Human peripheral blood T cells
Human peripheral blood mononuclear cells were obtained by leukapheresis of normal volunteers from the Department of Transfusion Medicine (without identifiers)42. Protocols were approved by the Institutional Review Board of the National Institutes of Health, Bethesda, MD. The cells were diluted in endotoxin-free PBS without Ca2+ or Mg2+ (BioWhittaker) and separated by density centrifugation using lymphocyte sedimentation medium (Organon Teknika Corp.) at 400 g for 30 min. The T cells in the mononuclear cell layer then were enriched by elutriation and stored in cold DMEM (BioWhittaker) until use.
Cell lines
Min6 murine insulin-secreting pancreatic beta cells, NCI-H345 and NCI-H82 human small cell lung carcinomas, AtT20 murine pituitary tumor cells, Jurkat human T cells and Vero monkey kidney epithelial cells were cultured in DMEM (Invitrogen, Grand Island, NY) containing 10% FCS, 2 mM L-glutamine, 1% penicillin/streptomycin (Invitrogen). Raw 264.7 murine macrophage cells were cultured in RPMI1640 (Invitrogen) with 10% FCS.
Polyreactive and monoreactive antibodies
Polyreactive and monoclonal antibodies were prepared from serum-free supernatants of cultured hybridoma cell lines as described11. In brief, 2E4 (IgM/λ), ZH-6 (IgM/κ), ZH-14 (IgG3/κ) and ZH-20 (IgM/κ) are all mouse monoclonal polyreactive antibodies. The heavy and light chain sequences of these antibodies are registered, respectively, in GenBank as: DQ913736.1/DQ986488.1, DQ913737.1/DQ986489.1, DQ913739.1/DQ986490.1, DQ913738.1/DQ986491.1. 2507 (IgM), 8512(IgM/κ) and 8018 (IgM) are murine monoclonal monoreactive antibodies (obtained from ATCC, Manassas, VA) and used as controls. 2507 is specific for E. coli O157( H7 O-antigen); 8512 reacts with bacterial cell wall peptidoglycan; and 8018 reacts with hepatitis B virus. Large amounts of antibody were prepared by culturing hybridoma cells in serum-free CD Hybridoma Medium (Invitrogen, Carlsbad, CA). Supernatants were concentrated and quantitated by Coomassie Plus Protein Assay Kit (Pierce Biotechnology, Inc, Rockford, IL).
Enrichment of polyreactive antibodies from human sera
Purified human IgM (>95% purification) isolated from normal human serum was obtained from Meridian Life Science, Inc., Memphis, TN. Human IgM was passed through and eluted from HiTrap Heparin HP columns (GE Healthcare, Piscataway, NJ) according to the manufacturer's instruction. The pass-through fractions were designated “polyreactive IgM-reduced” and the eluted fractions “polyreactive IgM-enriched.” IgM was eluted by washing with 2 M NaCl and desalted with HiTrap Desalting columns (GE Healthcare). The polyreactive-enriched and polyreactive-reduced IgM fractions were concentrated by Amicon® Pro Purification System (EMD Millipore, Billerica, MA). The protein concentration was determined by BCA protein assay (Pierce Biotechnology, Inc., Rockford, IL) and anti-human IgM ELISA assay. The polyreactivity of enriched IgM fraction was validated by binding to β-galactosidase, single stranded DNA and human insulin (data not shown) as described previously11.
Other antibodies and reagents
Recombinant human IL-2, FITC anti-human CD3, PECy7-conjugated anti-human CD4, FITC Annexin V, propidium iodide (PI), 7-amino-actinomycin D (7-AAD), anti-mouse IgM-PE, biotin-labeled anti-mouse IgM, APC streptavidin, PE streptavidin and Texas Red (TxRd) streptavidin were all purchased from BD Biosciences ( San Jose, CA). Streptavidin-conjugated Qdot 705 was obtained from Invitrogen (Carlsbad, CA). Draq5 was purchased from Axxora, LLC (San Diego, CA). Biotin-labeled anti-gp120 was purchased from ABcam (San Francisco, CA). PKH26 red fluorescent cell linker kit and PKH67 green fluorescent cell linker kit were purchased from Sigma (St. Louis, MO). Guinea pig serum containing complement was purchased from Cedarlane Laboratories (Ontario, Canada); Human serum containing complement was purchased from Innovative Research (Novi, MI).
Induction and detection of apoptosis
Primary cells or cell lines were washed twice with cold PBS containing 2 mM EDTA and re-suspended at 108 cells/ml. The cells then were seeded in 15 cm Petri dishes (BD Falcon) and irradiated for different time periods by exposure to a UV lamp with an output of 2 J/Cm2 (UVP, Upland CA). UV-treated cells then were washed with cold PBS once and cultured or stained for apoptosis and antibody binding. Apoptosis was determined by incubating the cells with FITC-labeled Annexin V (5 μg/100 μl) and 7-AAD (10 ng/100 μl) or propidium iodide (PI, 5 μg/100 μl).
T cell blast and HIV infection
Freshly isolated human peripheral blood T cells were cultured at 1 × 106/ml in DMEM containing 10% FCS, 10 units/ml rIL-2 and 1% PHA (Invitrogen). Culture medium was changed every other day. On day 5, the blasted T cells were divided into two separate flasks. One flask was infected with HIV-1IIIB (HIV) at 1–2 × 103/TCID50/ml (Advanced Biotechnologies, Inc.) in DMEM containing 10% FCS and 10 units/ml rIL-2. The second flask contained culture medium without HIV and served as the control. To measure viral infectivity, supernatants were collected at various times and replaced with fresh media. Infectivity in collected supernatants was determined by measuring p24 antigen using HIV-1 p24 ELISA kits (PerkinElmer) with data presented as mean ± SD43. On day 7 or 8 after HIV infection, T cells were harvested and suspended in cold PBS with 2% BSA and stained for apoptosis and antibody binding.
Binding of polyreactive antibody to apoptotic cells
To assess the binding of polyreactive versus monoreactive antibodies, 2 × 106 control, HIV-infected, live or UV-induced apoptotic cells were preincubated with 1% BSA at 4°C for 30 min to minimize the effect of nonspecific binding sites. The cells then were incubated with mouse monoclonal polyreactive or monoreactive antibodies (50 μg/ml) at 4°C for 30 mins, washed twice with cold PBS (with 1% BSA), re-suspended in PBS and incubated with PE-labeled goat anti-mouse IgM, PE-Cy7-anti-CD4 or biotin-anti-gp120 (revealed by TxRd streptavidin) for 30 min at 4°C. The cells then were washed and re-suspended in Annexin V binding buffer (BD Biosciences) and incubated with FITC Annexin V and TxRd-conjugated streptavidin for 30 min. Unstained cells and single-color stained cells were prepared and used for fluorescence compensation in flow cytometry and ImageStream experiments. Samples were fixed with 4% paraformaldehyde.
Binding of complement and measurement of C5a
Human serum containing complement was preadsorbed by four rounds of incubation with UV-treated T cells to eliminate immunoglobulin that might be present in the complement- containing serum that could bind to apoptotic cells.
UV-treated apoptotic human peripheral T cells were incubated with polyreactive or monoreactive monoclonal antibodies for 30 min, followed by incubation at 37°C for 5 minutes with a 1:20 dilution of human complement and then immediately cooled on ice and centrifuged at 4°C. Supernatants were collected and saved at −80°C for measuring C5a. The antibody and complement-treated cells then were washed in cold PBS, incubated with PE-labeled murine anti-human C3 monoclonal antibody (Cedarlane Laboratories) and the binding of complement was analyzed by LSRII flow cytometer. C5a levels were determined using the BD Cytometric Bead Array (CBA) Human Anaphylatoxin Kit according to the manufacture's instructions (BD Bioscience, San Jose, CA).
In vitro phagocytosis of UV-induced apoptotic cells
Cultured Raw 264.7 macrophages were harvested by gentle scraping of plates with Cell Scrapers (Corning Incorporated, Corning, NY), washed twice with DMEM, re-suspended in Diluent C and labeled with red fluorescent aliphatic dye PKH26 (Sigma) according to the manufacturer's instructions. Stained Raw cells then were re-suspended in Macrophage-SFM medium (Invitrogen) without FCS at 1 × 106 cells/ml in 12-well culture plates (Corning) overnight. The next day, freshly isolated mouse thymocytes or human peripheral T cells were treated with UV (2J/Cm2) for 6 min, washed, re-suspended with Diluent C and labeled with green fluorescent aliphatic dye PKH67 (Sigma). The UV-induced apoptotic cells then were incubated with monoreactive or polyreactive antibodies or PBS-BSA at 4°C for 30 min, incubated with a 1:10 dilution of adsorbed guinea pig or human complement for 5 min at 37°C, immediately washed in cold PBS and then added to the cultures containing PKH26-labeled Raw 264.7 macrophages. The cells were harvested at different time intervals, fixed with 4% paraformaldehyde and analyzed by flow cytometry by gating for green/red double positive cells (i.e., apoptotic cells phagocytosed by macrophages). Unstained cells and single stained cells (T cells or macrophages) were used for fluorescence compensation.
In vivo phagocytosis of UV-induced apoptotic cells
For in vivo phagocytosis experiments, 1 nM of PKH26 in 0.5 ml of Diluent B was injected ( i.p.) into μMT mice to stain peritoneal cavity macrophages 24 hours before injection of apoptotic target cells. Thymus cells from 4–6 week old C57BL mice were treated with UV for 6 min and stained with PKH67 followed by treatment with polyreactive or monoreactive antibodies and complement as described above. 3 × 106 antibody and complement-treated cells in 0.5 ml PBS then were injected into the peritoneal cavity of μMT mice that had been previously injected with PKH26. Mice were euthanized 30 to 90 min after injection and peritoneal cavity cells were harvested, washed with cold PBS and fixed with 4% paraformaldehyde. Phagocytosis was analyzed by flow cytometry and ImageStream.
Phagocytosis of HIV-induced apoptotic cells
To study phagocytosis of HIV-induced apoptotic cells by macrophages, human peripheral T cells were blasted by incubation with 10 units/ml rIL-2 and 1% PHA (Invitrogen) for 4 days as described above, labeled with green fluorescent aliphatic dye PKH67 and cultured for one more day. On day 5, the PKH67-labeled blasted T cells were divided into two flasks. In one flask HIV-1IIIB (HIV) at 1–2 × 103/TCID50/ml in serum-free medium (SFM) was added together with 10 units/ml rIL-2. In the second flask, culture medium without HIV served as the control. On day 7 or 8 after HIV infection, T cells were harvested and suspended in cold PBS with 2% BSA and treated with polyreactive or monoreactive antibodies for 30 min at 4°C followed by a 1:10 dilution of complement for 5 min at 37°C as described above. The above treated T cells then were added into PKH-26- labeled Raw264 macrophages, incubated for 30 min, harvested and fixed with 4% paraformaldehyde. Phagocytosis was analyzed by flow cytometry and ImageStream.
Flow cytometry
Antibody binding, complement binding, the C5a CBA test, apoptosis and phagocytosis were evaluated by flow cytometry. In general, single fluochrome (i.e., FITC, PE, APC, APC-Cy7, TxRd, 7AAD, PKH67, PKH26) stained samples and unstained samples were used for fluorescence compensation. Antibody isotope controls and unstained samples served as negative controls. 104 to 106 cell events were collected by FACSCalibur or LSRII (BD, San Jose, CA) and fluorescence intensity was determined by FlowJo software (TreeStar Inc., Ashland, OR).
ImageStream analysis
For antibody binding localization, six minute UV-treated human peripheral T cells were incubated with polyreactive antibody 2E4 or monoreactive antibody 8512, followed by staining with PE-conjugated anti-mouse IgM antibody. FITC-conjugated anti-human CD3 monoclonal antibody (BD Bioscience) was added to locate the membrane of cells. The cells then were fixed with 4% paraformaldehyde and stored at 4°C. Before analysis 0.25 μg Draq5 (to locate the nucleus binding) was added to 2 × 106 cells/100 μl PBS and incubated at room temperature for 20 min. Cell images, at about 50 cells per second were collected with IS100 ImageStream system (Amnis Corporation, Seattle, WA) and analyzed using ImageStream Data Analysis and Exploration Software (IDEAS, Amnis)44. Single fluorochrome-binding cells were collected and used for fluorescence compensation. Colocalization of fluorescent probes (i.e., bright detail similarity R3 feature of Channel 3 CD3-FITC signal vs. Channel 4 2E4-PE signal and Channel 4 2E4-PE signal vs. Channel 6 draq5 signal) was used to analyze the antibody binding localization in cell membrane, nucleus and cytoplasm.
Phagocytosis was evaluated by ImageStream analysis. During data collection and analysis, channel 2 was assigned to bright field signal, channel 3 to PKH 67 green fluorescence signal, channel 4 to PKH26 red fluorescence signal and channel 5 to TxRd fluorescence signal for antibody binding to HIV-gp120 antigen. Channel 6 was assigned to either draq 5 nucleus binding or to streptavidin-Qdot 705 (Invitrogen) for tracking polyreactive IgM binding in different stages of apoptosis using the markers Annexin V-FITC and 7-AAD. For phagocytosis of HIV-infected apoptotic T cells, dual lasers (i.e., 488 nm and 634 nm) were used for activation of the fluorochromes. HIV infection was evaluated using biotin labeled anti-gp120 followed by streptavidin-APC, assigned to channel 6. Single fluorochrome-stained cells were collected and assigned to each channel for fluorescence compensation by analysis with IDEAS software. Gated PKH26 (i.e., macrophage) positive cells that also were PKH67 positive were scored as phagocytosed. Delta centroid XY analysis by ImageStream is used to measure the distance between the centers of two fluorescent probes (i.e., PKH26 and PKH67). In the current experiment, phagocytosis was defined as: Delta centroid XY 0 to 2 μm as fully phagocytosed; 3 to 6 μm as partially phagocytosed; and 6 to 9 μm as adherent given the average T cell size of 5 to 8 μm and macrophage size of 10 to 15 μm. Images showing target cells binding to macrophages without real engulfment (adherence) also were considered positive being deemed the initiation of cell engulfment.
Statistical analysis
Duplicate samples were averaged and experiments were repeated at least three times. In the case of human peripheral blood T cells, three different donors were used. In the in vivo mouse experiments, each group consisted of three mice. Data are presented as mean ± standard error of the mean. Difference between groups was subjected to paired two-tailed Student's t tests. Significance was set at a p-value of ≤ 0.05.
References
Tauber, A. I. & Podolsky, S. H. The Generation of Diversity: Clonal Selection Theory and the Rise of Molecular Immunology, (Harvard University Press, Cambridge, MA, 1997).
Satoh, J., Prabhakar, B. S., Haspel, M. V., Ginsberg-Fellner, F. & Notkins, A. L. Human monoclonal autoantibodies that react with multiple endocrine organs. N Engl J Med 309, 217–220 (1983).
Casali, P. & Notkins, A. L. Probing the human B-cell repertoire with EBV: polyreactive antibodies and CD5+ B lymphocytes. Annu Rev Immunol 7, 513–535 (1989).
Coutinho, A., Kazatchkine, M. D. & Avrameas, S. Natural autoantibodies. Curr Opin Immunol 7, 812–818 (1995).
Notkins, A. L. Polyreactivity of antibody molecules. Trends Immunol 25, 174–179 (2004).
Prabhakar, B. S., Saegusa, J., Onodera, T. & Notkins, A. L. Lymphocytes capable of making monoclonal autoantibodies that react with multiple organs are a common feature of the normal B cell repertoire. J Immunol 133, 2815–2817 (1984).
Dighiero, G. et al. Murine hybridomas secreting natural monoclonal antibodies reacting with self antigens. J Immunol 131, 2267–2272 (1983).
Boes, M., Prodeus, A. P., Schmidt, T., Carroll, M. C. & Chen, J. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J Exp Med 188, 2381–2386 (1998).
Ochsenbein, A. F. et al. Control of early viral and bacterial distribution and disease by natural antibodies. Science 286, 2156–2159 (1999).
Quan, C. P., Berneman, A., Pires, R., Avrameas, S. & Bouvet, J. P. Natural polyreactive secretory immunoglobulin A autoantibodies as a possible barrier to infection in humans. Infect Immun 65, 3997–4004 (1997).
Zhou, Z. H. et al. The broad antibacterial activity of the natural antibody repertoire is due to polyreactive antibodies. Cell Host Microbe 1, 51–61 (2007).
Mouquet, H. & Nussenzweig, M. C. Polyreactive antibodies in adaptive immune responses to viruses. Cell Mol Life Sci 69, 1435–1445 (2012).
Ichiyoshi, Y., Zhou, M. & Casali, P. A human anti-insulin IgG autoantibody apparently arises through clonal selection from an insulin-specific "germ-line" natural antibody template. Analysis by V gene segment reassortment and site-directed mutagenesis. J Immunol 154, 226–238 (1995).
Wardemann, H. et al. Predominant autoantibody production by early human B cell precursors. Science 301, 1374–1377 (2003).
Zhou, Z. H., Tzioufas, A. G. & Notkins, A. L. Properties and function of polyreactive antibodies and polyreactive antigen-binding B cells. J Autoimmun 29, 219–228 (2007).
Haspel, M. V. et al. Multiple organ-reactive monoclonal autoantibodies. Nature 304, 73–76 (1983).
Chen, Z. J. et al. Polyreactive antigen-binding B cells are the predominant cell type in the newborn B cell repertoire. Eur J Immunol 28, 989–994 (1998).
Zhou, Z. H. & Notkins, A. L. Polyreactive antigen-binding B (PAB-) cells are widely distributed and the PAB population consists of both B-1+ and B-1− phenotypes. Clin Exp Immunol 137, 88–100 (2004).
Renehan, A. G., Booth, C. & Potten, C. S. What is apoptosis and why is it important? BMJ 322, 1536–1538 (2001).
Notley, C. A., Brown, M. A., Wright, G. P. & Ehrenstein, M. R. Natural IgM is required for suppression of inflammatory arthritis by apoptotic cells. J Immunol 186, 4967–4972 (2011).
Nagata, S., Hanayama, R. & Kawane, K. Autoimmunity and the clearance of dead cells. Cell 140, 619–630 (2010).
Gronwall, C. et al. IgM autoantibodies to distinct apoptosis-associated antigens correlate with protection from cardiovascular events and renal disease in patients with SLE. Clin Immunol 142, 390–398 (2012).
Grimsley, C. & Ravichandran, K. S. Cues for apoptotic cell engulfment: eat-me, don't eat-me and come-get-me signals. Trends Cell Biol 13, 648–656 (2003).
Ehrenstein, M. R. & Notley, C. A. The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol 10, 778–786 (2010).
Fu, M. et al. Identification of poly-reactive natural IgM antibody that recognizes late apoptotic cells and promotes phagocytosis of the cells. Apoptosis 12, 355–362 (2007).
Kim, J. Identification of a human monoclonal natural IgM antibody that recognizes early apoptotic cells and promotes phagocytosis. Hybridoma (Larchmt) 29, 275–281 (2010).
Quartier, P., Potter, P. K., Ehrenstein, M. R., Walport, M. J. & Botto, M. Predominant role of IgM-dependent activation of the classical pathway in the clearance of dying cells by murine bone marrow-derived macrophages in vitro. Eur J Immunol 35, 252–260 (2005).
Munoz, L. E., Lauber, K., Schiller, M., Manfredi, A. A. & Herrmann, M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat Rev Rheumatol 6, 280–289 (2010).
Gaipl, U. S. et al. Clearance deficiency and systemic lupus erythematosus (SLE). J Autoimmun 28, 114–121 (2007).
Elmore, S. Apoptosis: a review of programmed cell death. Toxicol Pathol 35, 495–516 (2007).
Henson, P. M. & Hume, D. A. Apoptotic cell removal in development and tissue homeostasis. Trends Immunol 27, 244–250 (2006).
Li, C. J., Friedman, D. J., Wang, C., Metelev, V. & Pardee, A. B. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science 268, 429–431 (1995).
Tomer, Y. & Shoenfeld, Y. The significance of natural autoantibodies. Immunol Invest 17, 389–424 (1988).
Grabar, P. Autoantibodies and the physiological role of immunoglobulins. Immunology Today 4, 337–340 (1983).
Stafford, P. et al. Physical characterization of the "immunosignaturing effect". Mol Cell Proteomics 11, M111 011593 (2012).
Shaw, P. X. et al. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance and protective immunity. J Clin Invest 105, 1731–1740 (2000).
Gronwall, C., Vas, J. & Silverman, G. J. Protective Roles of Natural IgM Antibodies. Front Immunol 3, 66 (2012).
Kaveri, S. V. Intravenous immunoglobulin: Exploiting the potential of natural antibodies. Autoimmun Rev 11, 792–794 (2012).
Sapir, T. & Shoenfeld, Y. Facing the enigma of immunomodulatory effects of intravenous immunoglobulin. Clin Rev Allergy Immunol 29, 185–199 (2005).
Bruley-Rosset, M. et al. Polyreactive autoantibodies purified from human intravenous immunoglobulins prevent the development of experimental autoimmune diseases. Lab Invest 83, 1013–1023 (2003).
Kaveri, S. V., Silverman, G. J. & Bayry, J. Natural IgM in immune equilibrium and harnessing their therapeutic potential. J Immunol 188, 939–945 (2012).
Ardans, J. A., Economou, A. P., Martinson, J. M., Jr, Zhou, M. & Wahl, L. M. Oxidized low-density and high-density lipoproteins regulate the production of matrix metalloproteinase-1 and -9 by activated monocytes. J Leukoc Biol 71, 1012–1018 (2002).
Moutsopoulos, N. M. et al. Regulation of the tonsil cytokine milieu favors HIV susceptibility. J Leukoc Biol 80, 1145–1155 (2006).
Phanse, Y. et al. Analyzing cellular internalization of nanoparticles and bacteria by multi-spectral imaging flow cytometry. J Vis Exp, e3884 (2012).
Acknowledgements
The authors thank Richard A. DeMarco of Amnis Corp for comments on ImageStream analysis. This research was supported by the Intramural Research Programs of the US National Institutes of Health and Food and Drug Administration. The views expressed in this manuscript represent the opinions of the authors and do not necessarily represent the official views of the NIH or FDA.
Author information
Authors and Affiliations
Contributions
Z.Z. and A.L.N. conceived of the study, analyzed the data and wrote the paper assisted by L.W., S.W. and S.K. Experiments were performed by Z.Z., T.W., Y.X., L.H.S., Y.Z. and L.Z.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplemental Fig.1 and Fig.2
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Zhou, Zh., Wild, T., Xiong, Y. et al. Polyreactive Antibodies Plus Complement Enhance the Phagocytosis of Cells Made Apoptotic by UV-Light or HIV. Sci Rep 3, 2271 (2013). https://doi.org/10.1038/srep02271
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep02271
This article is cited by
-
Oxidized hemoglobin triggers polyreactivity and autoreactivity of human IgG via transfer of heme
Communications Biology (2023)
-
Stimulation of Toll-Like Receptors profoundly influences the titer of polyreactive antibodies in the circulation
Scientific Reports (2015)
-
The protective role of immunoglobulins in fungal infections and inflammation
Seminars in Immunopathology (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.