Abstract
In heart transplantation, selection of an optimal recipient-donor match has been constrained by the lack of individualized prediction models. Here we developed a customized donor-matching model (CODUSA) for patients requiring heart transplantations, by combining simulated annealing and artificial neural networks. Using this approach, by analyzing 59,698 adult heart transplant patients, we found that donor age matching was the variable most strongly associated with long-term survival. Female hearts were given to 21% of the women and 0% of the men and recipients with blood group B received identical matched blood group in only 18% of best-case match compared with 73% for the original match. By optimizing the donor profile, the survival could be improved with 33 months. These findings strongly suggest that the CODUSA model can improve the ability to select optimal match and avoid worst-case match in the clinical setting. This is an important step towards personalized medicine.
Similar content being viewed by others
Introduction
Heart transplantation is the only option for survival for selected patients with end-stage heart failure. In recent years, survival after heart transplantation has improved significantly. Reasons for improved outcome include refinement of donor and recipient selection methods, better donor organ preservation, lower perioperative mortality rates and enhanced immunosuppressive protocols1. The median survival is now more than 10 years2. However, donor scarcity is a major issue. In addition, suboptimal recipient-donor match may lead to acute rejection and cardiac allograft vasculopathy, leading to early and late mortality. Much research has been directed towards identifying factors predicting survival and optimal organ utilization.
Organ matching is primarily based on ABO blood group compatibility and patient size. Other factors such as gender, allograft ischemia time, medical conditions prior to transplant and human leukocyte antigen (HLA) mismatch have all been implied as risk factors for acute rejection but may not be used in the organ matching2,3,4,5. Scant information exists concerning predictors of long-term survival after heart transplantation6. The long-term outcome depends on several factors and it is important to recognize that the influence from some of these varies over time. Because of the inherent complexity of coupled nonlinear biological systems, the development of computational models may be necessary for achieving a quantitative understanding of the outcome7. More complex analyses, including the interaction between several of the variables, are lacking and no study has to date created a personalized recipient donor matching model for identifying worst-case match and to find best possible match in heart transplantation. Instead of using the traditional methodology based on standard linear model with the assumption of proportional hazards to predict the survival, a more flexible non-linear survival model based on artificial neural networks (ANNs) is preferable8. Despite computational learning approaches being well suited for medical application there are only a few examples within the field of transplantation9.
The simulated annealing (SA) algorithm can be found in various research fields for parameter optimization. The SA algorithm developed by Kirkpatrick et al.10 has been previously applied to a wide range of medical problems, including microbial engineering, classification of cancer gene expression data and gamma knife planning11,12,13. SA is a random-search optimization technique for finding the global minimum of a cost function. SA is inspired by the physical process of controlled cooling of solid materials.
Here we show that SA could be used to ‘customize’ an optimal donor for the heart transplant recipients on the waiting list, which makes it possible to avoid poor recipient-donor match and identify the best possible match.
Results
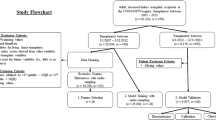
A total of 62,114 recipients of heart transplants from deceased donors were collected from the International Society for Heart and Lung Transplantation (ISHLT) Registry during the 19-year study period. After case records with incomplete mandatory data were excluded, the final study population comprised 59,698 patients. The mean age of the overall cohort was 51 ± 11 years and 20% were women. The patient material comprised 318,904 patient-years, with a mean follow-up duration of 5.3 ± 4.6 years (median 4.5, range 0–19.8 years). The most common indication for heart transplantation and cause of end-stage heart failure were ischemic cardiomyopathy in 26,266 patients (44%) and non-ischemic cardiomyopathy in 26,047 patients (44%). The average donor age was 32 ± 13 years. The majority of donors were men (69%). The study material was randomly divided in a derivation cohort (N = 49,856) and an independent validation cohort (N = 9,842). The used variables in the model development were based on which information is available at the time of transplantation (Table 1A–B). The derivation and the validation cohorts were generally similar although a higher proportion of symptomatic peripheral vascular disease was registered in the validation group.
Calibration and discrimination of the ANN survival model
The applied ANN model (a generalization of the standard Cox proportional hazard model) is a flexible non-linear survival models. To improve the performance we used an ensemble (Committee Machine) of ANNs instead of a single prediction model14,15. We optimized the model by ranking and minimizing the number of risk variables, similar to a stepwise backwards elimination procedure (Figure 1A). The final model included 30 of the 38 candidate variables corresponding to 41 inputs. The hazard ratios (HR) for the top confounding risk factors, ranked in order of influence upon discriminatory power, are presented together with the mandatory variables in Table 2. The 12 mandatory variables (age, blood group, gender, height and weight ratio) were not included in the ranking because we wanted them incorporated in the ANN model regardless of discriminatory power. We validated the model by predicting the cumulative survival for each patient in the validation cohort. The Harrell's Concordance index for the ANN model was 0.59 (95% CI, 0.586–0.594) and the difference in area under the predicted survival curve was 1.4% compared with the Kaplan-Meier survival curve (Figure 1B).
Schematic illustration of the variable-ranking process for ANN training and validation.
Panel (A): Step I: training of the survival model using fivefold cross-validation and a committee machine with 8 members. Step II: variable ranking using the trained survival model from step I. Each variable is omitted from the model and the decrease in performance is recorded. The variable resulting in the least reduction in performance is removed. Steps I–II are repeated until only one variable is left. A ranking list is constructed using the elimination order. To evaluate whether the final ANN risk prediction model was applicable in a patient cohort that had not been used in the development of the ANNs, the validation set (N = 9,842) was used as a blind test. Panel (B): Heart transplantation prediction of conditional failure probability. The solid grey line presents the predicted mortality in the validation cohort using the ANN survival model. The dashed black line presents the observed mortality for the validation cohort (Kaplan-Meier estimate). The predicted mortality obtained with our pilot Simulated Annealing (SA) model is presented with a blue line, Virtual Recipient Donor Match (VRDM) model with a red line. Best match is presented with solid line and worse match with dashed line.
Customize an optimal donor using simulated annealing
We used the SA to ‘customize’ an optimal and worst donor, respectively, for the recipients. The main feature of the SA algorithm is the ability to avoid being trapped in local minimum. This is done by letting the algorithm accept not only better solutions but also worse solutions, “uphill moves”, with a given probability16. The SA algorithm is expressed by a flowchart in Figure 2A. We tested the SA algorithm for selecting best-case match (N = 36,696) and worst-case match (N = 34,638) using the five donor variables: age, gender, blood group, height and weight. The area under the predicted survival curve (AUPSC) was calculated for each recipient-donor match. Figure 2B shows how much the AUPSC changes as one of the five donor variables is altered. Figure 2C shows the monitoring of the SA function that is looking for a best recipient-donor match for one randomly selected patient from the registry. At approximately 7,000 iterations, the temperature was close to zero and an (approximate) optimum set of input values (the donor profile) was found. In Figure 3A, a plot of a random selection of 1,000 patients and their respective predicted survival curve from the experiment is drawn based on the best-case donor variables. The corresponding plot for worst-case match is shown in Figure 3B.
Recipient donor match using simulation annealing (SA).
Panel (A): The simulated annealing (SA) Monte Carlo algorithm: A recipient is randomly selected from the recipient registry. The predicted survival is calculated and the existed recipient-donor profile and the data are recorded. Then the simulation process is started. A donor variable and the value for this variable are selected at random. The difference (delta) between the predicted survival for this new recipient donor profile and the earlier best survival is calculated. If the predicted survival is better than the previous, the best recipient-donor profile and the best-predicted survival are updated. If not the test for allowance “uphill” moves are performed. The allowance for "uphill" moves saves the method from becoming stuck at local minima, which means that a worse value can be accepted in some cases. This procedure is repeated 50 times and then the temperature is decreased with 4%. The whole procedure is started with 50 new trials at this new temperature. When the temperature has gone below 0.000025 the simulation for this patient is ended and a new is selected. The waiting list is updated with a new recipient and the procedure is repeated. Panel (B): Area under predicted survival curve (AUPSC) related to change in one of the SA variables. Panel (C): Monitoring of the simulated annealing (SA) function as it is looking for a best-case match.
Customize Optimal Donor Using Simulated Annealing (CODUSA).
Panel (A): Random selection of 1,000 patients and their respective survival curve based on the best case customized donor profile. Panel (B): Random selection of 1,000 patients and their respective survival curve based on the worse case customized donor profile.
Donor age was the variable that influenced outcome at most of the five evaluated donor variables. All patients received extreme values. For best-case match the optimal donor age was 15 ± 0.0 years compared with 32 ± 13 years, P < 0.001, for the original recipient-donor match. For the worse case match the donor age was 80 ± 0.0 years compared with 32 ± 13 years, P < 0.001, for the original recipient-donor match. Furthermore, in the best-case match only 57 female hearts were given to 29,295 men while 1,568 (21%) female hearts were given to 7,384 women. Thus, not all women received a female donor organ. In the worst-case match, 27,652 (100%) female hearts were given to 27,668 men and 6,699 (96%) female hearts to 6,970 women, Table 3.
The blood group matching was analysed and differences between optimal and suboptimal outcomes were studied. Controversially, recipients with blood group B received identical matched blood group in only 18% of best-case match and 68% in worst-case match compared with 73%, P < 0.001 for the original recipient-donor match. We observed a similar finding for recipient with blood group AB. The SA algorithm avoided donor blood group B in the best-case match. Only 2% of these recipients were matched to donors with blood group B compared with 24%, P < 0.001, for the original recipient-donor match, Table 3.
The last of the five studied variables, weight ratio, showed a similar result as donor age for the best-case match. 65% of the recipients received an extreme value of a recipient-donor ratio of 0.5. For the recipients with non-extreme values (N = 12,682) the optimal recipient donor weight ration was 0.83 ± 0.24. We could not see the same results for the recipient donor height ratio. In the best-case match none of the recipients achieved any extreme value. Instead, the optimum ratio was 1.08 ± 0.10. However, in the worst-case match the recipient donor height ration was 1.5 in 64% of the cases and 0.5 in 36% of the cases, Table 3.
Prediction of conditional failure after heart transplantation
Figure 1B illustrates the mean value for all best-case and worst-case matches. By optimizing the donor profile, the survival could be improved with 33 months. In order to evaluate if the SA had been able to improve the survival compared to “real-life” donor profiles, we performed a virtual recipient-donor match (VRDM). The recipient was matched using all the different donor profiles from the registry. The recipient-donor combination with best-predicted survival was chosen. The procedure was repeated for all recipients and the mean predicted survival curve for all best-case matches was plotted. We identified the worst combination in a similar way. Of note, even if we created artificial combinations by changing the five variables, we could not improve survival compared to using VRDM combinations. On the other hand, we could identify much worse combinations using SA, Figure 1B.
Discussion
Heart transplantation remains the therapy of choice for end-stage heart failure. There is shortage of donors and many candidates die awaiting transplantation. Simulation-based analysis offers a practical way to make informed decisions by predicting likely outcomes from different choices17. To our knowledge, this study represents the first to show how to utilize SA in heart transplantation. SA is a stochastic optimization technique for non-linear modeling. SA has been used in medical research to solve optimization problems and has gained renewed interest since its introduction in the 1980s10,11,12,13. Furthermore, ANNs have become a very powerful and practical method to model very complex non-linear systems. However, the published experience with ANNs within transplantation research is limited. Only a few studies have been published in kidney, liver and heart transplantation9. In this study, we demonstrated that by combining SA and ANNs, a customize optimal donor using simulated annealing (CODUSA) model for patients requiring heart transplantation can be developed that is accurate and highly reproducible, allowing for identification of optimal and even more important worst-case recipient-donor match.
In the present study, after the selection of influential variables by the SA model, some variables remained different between the optimal and sub-optimal matches. This finding suggests that best-case and worst-case matches are governed by different sets of factors. Thus, some recipient-donor combinations must be avoided at all times while some combinations of characteristics must be considered in every transplantation attempt. The strength of our SA model lies in the identification of unsuitable donors for a specific recipient. To our knowledge, this is the first study of its kind and a step towards so called ‘personalized medicine’ in the field of heart transplantation. Future studies, should include more donor variables, but may also include genomic and proteomic data to increase the predictive ability of the SA model.
During the last decades, several studies have evaluated the importance of the matching criteria age, ischemia time, ABO blood group and body size. The influence of age on survival is well established. Older donors and recipients are associated with atrial fibrillation, cardiac allograft vasculopathy and higher mortality18,19. The importance of donor age was confirmed in the present study and all recipients in the CODUSA model were matched to a low age donor.
The long-term effect of gender is a matter of controversy. Our data showed that female hearts should not be given to men, but the SA model did also prefer male donor to female recipients. Generally, heart transplant patients with donor-recipient sex mismatch have inferior survival3,20,21. But, for female recipient this is controversial. Weiss et al showed, analyzing the UNOS registry, that no survival advantage was seen for women receiving organ with same sex22. Many mechanisms explaining these discrepancies have been proposed, but remain to be fully elucidated. For example, it has been suggested that there may be immunologic or hormonal factors involved20. Gender differences in the susceptibility to ischemia reperfusion injury have also been suggested and at any given duration of cold ischemia, heart transplants coming from female donors had consistently worse graft survival compared with male donors20.
We found no significant difference in survival following compatible versus identical ABO matching. Similar findings have been reported in previous studies using donor hearts with compatible, but non-identical, blood group match23,24,25. However, our results indicate that donor blood group B is not selected and these patients are instead given donor blood group 0. A similar finding was seen for the recipients with blood group AB. Also here donors with blood group B were avoided. Already in the series from Barnard's group26, reduced survival in recipients with the B antigen was seen. It has been hypothesized that patients with blood group B may elicit a greater immune response but this remains controversial27.
The present study demonstrated that weight and height have been handled differently depending on if it is best-case match or worst-case match. Previous data suggest that the donor-recipient weight match criteria may be extended to increase the donor pool28,29. However, height has been proposed to be a better predictor of heart size as estimated by left ventricular end-diastolic diameter, as well as a novel measurement from the superior vena cava-right atrium junction to inferior vena cava-right atrium junction30. Here we show that the optimal recipient-donor weight ratio is around 0.8, but a recipient-donor height ratio should be 1.08. Both extreme undersize and oversize in height were correlated with reduced survival.
The limitations of the present study deserve further discussion. The complexity between different risk variables from two individuals is extreme. Certain variables have historically been considered more important than others and we have therefore focused on these (blood group, gender, age, weight, height) due to temporal constraints. Each simulation usually spanned several hours, which limited the study. It took 30 days à 24 hours for the cluster to calculate the results of approximately 35,000 patients. In one experiment, we identified best-case match and in another experiment worst-case match. Ischemia time was not included. Ideally we would have included more donor variables than the 5 utilized in our study.
In conclusion, by applying ANNs, we have designed an effective model for predicting long-term outcomes in patients undergoing heart transplantation that can incorporate multiple recipient and donor-related variables. A CODUSA model based on SA shows strong potential to enhance the prediction of patient survival and to identify important variables that have an impact on individual matching outcome. Information provided by our CODUSA model may improve the ability to select optimal match and avoid worst-case match in the clinical setting, functioning as an alternative to prospective cross-matching.
Methods
Data source
In this registry study, we used the International Society for Heart and Lung Transplantation (ISHLT) registry (www.ishlt.org), the largest repository of heart transplant data in the world2.
The registry includes data since 1983 and at present, over 223 centers from 18 countries submit data to this registry. Heart donors and the corresponding recipient transplanted between January 1, 1988 and December 31, 2006 were included in the study population. The latest yearly follow up was on January 19, 2008. We excluded patients if they were younger than 18 years at transplantation or have incomplete mandatory data (diagnosis, blood group, age, gender, follow up duration and/or cause of death). A subset of 38 clinically relevant variables, of the registered 293, were selected, Table 1. The Ethics Committee for Clinical Research at Lund University, Sweden approved the study protocol.
Design of ANN
The applied ANN survival model follows the principles described by Biganzoli et al., with the extension of using an ensemble of ANNs instead of a single prediction model31 (Supplementary Methods). The number of hidden nodes was determined based on experiments starting with a single node and increasing the number of nodes until the highest accuracy was found for the development set. The selection of risk variables to use as inputs was achieved using a backward elimination technique removing low ranking risk variables, Figure 1A. To improve the generalization performance weight-decay regularization, optimized using a 5-fold cross-validation technique, was used in the model building process. The number of ANN models used in the ensemble was 8 and no effort was made to tune this number. The ensemble output was the mean of the 8 individual ANN model32.
Time dependent hazard ratio
The time dependent hazard ratios for the risk variables were determined in a similar way as described by Lippmann and co-workers33 (Supplementary Methods). By changing the risk variable in a patient from absent to present and calculating the hazard for the two conditions at each time interval, a time dependent hazard ratio for the specific risk variable of each patient could be determined. A hazard ratio for the specific variable was then obtained by computing the geometric mean of the hazard ratio from all patients. The 95% confidence intervals hazard ratio was calculated using the bootstrap technique (N = 10,000).
SA algorithm
The SA is the metallurgical process of heating up a solid material and cooling it slowly until it crystallizes. Atoms have high energies at very high temperatures, which give them freedom in their ability to restructure themselves. When the temperature is reduced the energy decreases, until a state of minimum energy for the atom is achieved. SA seeks to emulate this process. SA begins at a high temperature where the input values are allowed to have a pronounced range of variation. As algorithm progresses temperature is allowed to fall, which restricts the inputs to vary. The algorithm will progress to a better solution, just as a metal achieves a better crystal structure through the annealing process. The main feature of SA algorithm is the ability to avoid being trapped in local minima by allowing for uphill moves of the cost function. SA begins at a high temperature, allowing for almost all uphill moves and new solutions are generated given a pre-defined range of variation for each input variable. A new solution is always accepted if it improves the cost function (downhill move) and with a certain probability if it worsens the cost function (uphill move). As the SA algorithm is running the temperature is gradually lowered, thereby making it harder and harder for uphill moves to occur. Finally, when a very low temperature has been reached the best obtained solution is extracted, which then, hopefully, is close to the optimal one.
The SA simulation started with selection of a starting temperature (t = 1). Five donor features were used to toggle (age, gender, blood group, weight and height). Which of the donor variables to be changed were randomly selected in each of the trial loops at a given temperature. For each temperature 50 trials were performed. The best recipient-donor combination was selected using the SA with acceptance of dis-improvements, or “uphill moves”. In the next step the temperature was decreased with 4% (k = 0.96) and the procedure was repeated. When the temperature was below 0.000025 the procedure was ended and a new recipient was chosen, Figure 2A.
Statistics
Values are reported as mean (s.d.) or median (i.q.r) for continuous variables and as percentages for categorical variables. Participant characteristics were compared using χ2-test for categorical variables and Wilcoxon test for continuous variables. Kaplan-Meier survival analysis was performed to demonstrate whether survival predictions from ANNs trained by the outcome data in a particular time point was capable of predicting survival for the entire follow-up period. Harrell's Concordance index34 was used to measure prediction performance in the survival analysis. Multiple and probability imputation was used to deal with missing data35,36 (Supplementary Methods).
High-performance computing clusters were used to train and evaluate the ANNs and performing the SA. The ANN calibration and analyses were performed with MatLab Distribution Computing Server 2010a, Neural Network Toolbox (MathWorks, Natick, Mass). Graphs and statistical analyses were performed using the Stata MP version 12.1 (2012) statistical package (StataCorp LP, College Station, TX) and R version 2.15.1 (2012, The R Foundation for Statistical Computing).
Data and materials availability
Requests for the data should be directed to the ISHLT Transplant Registry (https://www.ishlt.org/registries/registriesDataRequest.asp). The developed neural network model, including weights (Matlab net files), can be obtained from the corresponding author.
References
Hunt, S. A. Taking heart--cardiac transplantation past, present and future. N. Engl. J. Med. 355, 231–235 (2006).
Stehlik, J. et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult heart transplant report--2010. J. Heart Lung Transplant. 29, 1089–1103 (2010).
Weiss et al. Impact of recipient body mass index on organ allocation and mortality in orthotopic heart transplantation. J Heart Lung Transplant 28, 1150–1157 (2009).
Russo, M. J. et al. The effect of ischemic time on survival after heart transplantation varies by donor age: an analysis of the United Network for Organ Sharing database. J. Thorac. Cardiovasc. Surg. 133, 554–559 (2007).
Smith, J. D., Rose, M. L., Pomerance, A., Burke, M. & Yacoub, M. H. Reduction of cellular rejection and increase in longer-term survival after heart transplantation after HLA-DR matching. Lancet 346, 1318–1322 (1995).
Kilic, A. et al. What predicts long-term survival after heart transplantation? An analysis of 9,400 ten-year survivors. Ann. Thorac. Surg. 93, 699–704 (2012).
Winslow, R. L., Trayanova, N., Geman, D. & Miller, M. I. Computational medicine: translating models to clinical care. Sci. Transl. Med. 4, 158rv111 (2012).
Krogh, A. What are artificial neural networks? Nat. Biotechnol. 26, 195–197 (2008).
Sousa, F. S. et al. Application of the intelligent techniques in transplantation databases: a review of articles published in 2009 and 2010. Transplant. Proc. 43, 1340–1342 (2011).
Kirkpatrick, S., Gelatt, C. D., Jr & Vecchi, M. P. Optimization by simulated annealing. Science 220, 671–680 (1983).
Salis, H. M., Mirsky, E. A. & Voigt, C. A. Automated design of synthetic ribosome binding sites to control protein expression. Nat. Biotechnol. 27, 946–950 (2009).
Kuiper, R. et al. A gene expression signature for high-risk multiple myeloma. Leukemia 26, 2406–2413 (2012).
Zhang, P., Dean, D., Metzger, A. & Sibata, C. Optimization of Gamma knife treatment planning via guided evolutionary simulated annealing. Med. Phys. 28, 1746–1752 (2001).
Biganzoli, E., Boracchi, P., Mariani, L. & Marubini, E. Feed forward neural networks for the analysis of censored survival data: a partial logistic regression approach. Stat. Med. 17, 1169–1186 (1998).
Hansen, L. K. & Salamon, P. Neural network ensembles. IEEE Trans. Pattern Anal. Machine Intel. 12, 993–1001 (1990).
Folino, G., Pizzuti, C. & Spezzano, G. Genetic programming and simulated annealing: a hybrid method to evolve decision trees. In Proceedings of the 3rd European Conference on Genetic Programming (eds. Poli, R.et al.), Vol. 1802, (Springer-Verlag, Edinburgh, Scotland; 2000).
Thompson, D. et al. Simulating the allocation of organs for transplantation. Health Care Manag. Sci 7, 331–338 (2004).
Nagji, A. S. et al. Donor age is associated with chronic allograft vasculopathy after adult heart transplantation: implications for donor allocation. Ann. Thorac. Surg. 90, 168–175 (2010).
Topkara, V. K. et al. Effect of donor age on long-term survival following cardiac transplantation. J. Card. Surg. 21, 125–129 (2006).
Zeier, M., Dohler, B., Opelz, G. & Ritz, E. The effect of donor gender on graft survival. J. Am. Soc. Nephrol. 13, 2570–2576 (2002).
Khush, K. K., Kubo, J. T. & Desai, M. Influence of donor and recipient sex mismatch on heart transplant outcomes: analysis of the International Society for Heart and Lung Transplantation Registry. J. Heart Lung Transplant. 31, 459–466 (2012).
Weiss, E. S. et al. The impact of donor-recipient sex matching on survival after orthotopic heart transplantation: analysis of 18 000 transplants in the modern era. Circ Heart Fail 2, 401–408 (2009).
Kocher, A. A. et al. Effect of ABO blood type matching in cardiac transplant recipients. Transplant. Proc. 33, 2752–2754 (2001).
Neves, C., Prieto, D., Sola, E. & Antunes, M. J. Heart transplantation from donors of different ABO blood type. Transplant. Proc. 41, 938–940 (2009).
Sjogren, J. et al. Heart transplantation with ABO-identical versus ABO-compatible cardiac grafts: influence on long-term survival. Scand. Cardiovasc. J. 44, 373–379 (2010).
Lanza, R. P., Cooper, D. K. & Barnard, C. N. Effect of ABO blood-group antigens on long-term survival after cardiac transplantation. N. Engl. J. Med. 307, 1275–1276 (1982).
Nakatani, T., Aida, H., Frazier, O. H. & Macris, M. P. Effect of ABO blood type on survival of heart transplant patients treated with cyclosporine. J. Heart Transplant. 8, 27–33 (1989).
Taghavi, S., Wilson, L. M., Brann, S. H., Gaughan, J. & Mangi, A. A. Cardiac transplantation can be safely performed with low donor-to-recipient body weight ratios. J. Card. Fail. 18, 688–693 (2012).
Patel, N. D. et al. Impact of donor-to-recipient weight ratio on survival after heart transplantation: analysis of the United Network for Organ Sharing Database. Circulation 118, S83–88 (2008).
Zuckerman, W. A., Richmond, M. E., Singh, R. K., Chen, J. M. & Addonizio, L. J. Use of height and a novel echocardiographic measurement to improve size-matching for pediatric heart transplantation. J. Heart Lung Transplant. 31, 896–902 (2012).
Ansari, D. et al. Artificial neural networks predict survival from pancreatic cancer after radical surgery. Am. J. Surg. 205, 1–7 (2013).
Nilsson, J. et al. Risk factor identification and mortality prediction in cardiac surgery using artificial neural networks. J. Thorac. Cardiovasc. Surg. 132, 12–19 (2006).
Lippmann, R. P. & Shahian, D. M. Coronary artery bypass risk prediction using neural networks. Ann Thorac Surg 63, 1635–1643 (1997).
Harrell, F. E., Jr, Califf, R. M., Pryor, D. B., Lee, K. L. & Rosati, R. A. Evaluating the yield of medical tests. Jama 247, 2543–2546 (1982).
Schafer, J. L. Multiple imputation: a primer. Stat Methods Med Res 8, 3–15 (1999).
Schemper, M. & Heinze, G. Probability imputation revisited for prognostic factor studies. Stat Med 16, 73–80 (1997).
Acknowledgements
The data have been provided by the ISHLT Transplant Registry. The interpretation and reporting of these data are the responsibility of the authors. We are grateful to Lund University NIC Application Research Center (www.lunarc.lu.se) for computational resources.
Funding: This study wasmade possible by grants from the Swedish National Infrastructure for Computing, Swedish Heart-Lung Foundation, Swedish Society of Medicine, Government grant for clinical research, Region Skåne Research Funds, Donation Funds of Lund University Hospital and the Crafoord Foundation.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study design and data interpretation. J.N. and M.O., undertook the analysis and validation of the data. J.N. performed the computer programming. D.A. and J.N. drafted the initial report and all authors contributed to the final draft. Data was provided from the ISHLT registry by staff at the US, United Network for Organ Sharing and compiled by J.N.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplemental Material
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Ansari, D., Andersson, B., Ohlsson, M. et al. CODUSA - Customize Optimal Donor Using Simulated Annealing In Heart Transplantation. Sci Rep 3, 1922 (2013). https://doi.org/10.1038/srep01922
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep01922
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.