Abstract
Over the past decade much progress has been made towards the treatment of disease with recombinant adeno-associated viral vectors, ranging from cancer to muscular dystrophies and autoimmune diseases to cystic fibrosis. Given inherent challenges of vector delivery we developed a system incorporating commercially available dialysis equipment. This concept was evaluated in vitro utilizing rAAV expressing the reporter gene human placental alkaline phosphatase. A number of pre-circulating conditions were assessed. Vector recovery was evaluated by quantitative vector genome analysis and cellular transduction assays. A dialysis circulation time course was established and results were recorded across varied conditions ranging from approximately 2 to 90% retention of viable vector. This approach is unique in that it focuses on efficient localized, isolated and continual delivery of vector to target tissues, provides for the preservation of tissue integrity with dialysis for metabolic exchange and allows for the transfer of oxygen through a secondary membrane post-dialysis.
Similar content being viewed by others
Introduction
AAV is a single-stranded DNA-containing virus, belonging to the Parvoviridae family that has not been shown to be associated with any known pathogenicity in humans1,2,3. At least 12 serotypes have been identified from primates4,5,6 displaying various tropisms in vivo7,8,9,10. rAAV vectors are attractive for gene therapy applications, given they are capable of transducing numerous cell types and support stable, long-term gene expression with minimal induction of an immune response11,12. Stable gene expression following rAAV injection into muscle has been reported for up to 2 years in mice and more than 7 years in dogs and rhesus monkeys13,14,15,16,17. The safety and efficacy of rAAV-based gene delivery approaches can depend not only on the serotype, but also on numerous other variables including the quality of the vector preparation, vector dose, route of delivery, anatomical barriers, animal species and host immune status18,19,20.
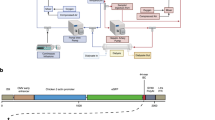
In order to overcome some of these limitations we have developed a regionalized dialysis-based extracorporeal system for rAAV vector delivery localized to the desired tissue bed ( Figure 1 ). Our system utilizes hemodialysis equipment that is already commercially available in the United States and readily available in most clinical settings. Over recent years, modern hemodialysis membranes, derived from synthetic polymers, have improved and are more biocompatible21. These membranes are widely used in patients with end stage kidney disease and are well tolerated clinically. In the present study, we utilized two different types of dialysis membranes, a low flux dialyzer composed of polysulfone (F3, Fresenius) and a high flux dialyzer composed of polyamide (6H, Gambro). The dialyzers utilized were chosen for their biocompatible properties and their small priming volume that limits the volume of blood to fill up the dialyzer. Despite the biocompatibility of the membranes, clinically negligible amounts of protein do bind to the membrane22,23. To our knowledge, adsorption of rAAV to dialysis membranes has not been studied. In this manuscript we describe the circuit and present in vitro data evaluating the effectiveness of vector transduction following the circulatory procedure.
Results
Four different priming solutions were evaluated: normal saline (0.9% sodium chloride), Pluronic F-68 (0.01% in saline), bovine serum albumin (5% in saline) and whole blood from a rAAV6 sero-negative non-human primate (m. nemestrina), a medium-sized Old World monkey. When utilizing saline alone to prime the circuit ( Figure 2 ) there was a rapid drop in the circulating concentration of AAV. The decline continued in a time dependent fashion until the end of the 60 min when little AAV was left in the sample fluid as assessed by quantitative PCR (qPCR). Transduction assays confirmed very little vector retention in the samples as early as 5 minutes into circulation. When using surfactant Pluronic F-68 at 0.01% in saline as the priming solution vector recovery over time remained poor ( Figure 3 ). The inclusion of a 5-minute pre-circulation of 5% bovine serum albumin into saline priming fluid of the extracorporeal circuit ( Figure 4A, 4B ) revealed improved vector retention and transduction throughout the duration of the circulatory period, though there was an initial drop in vector genome retention, as determined by qPCR, shortly after starting the procedure. Increasing the pre-dialysis incubation time of the albumin priming fluid to one hour ( Figure 4C ) showed further improvement in vector recovery during the re-circulation procedure. Priming with blood was highly effective at preventing rAAV6 binding to the circuit components as well preserving transduction capacity throughout the circulation procedure ( Figure 5 ).
Evaluation of rAAV6 vector genome retention and viability following 1 hour of circulation through the dialysis circuit in the presence of saline alone.
(A) Vector genome quantification as determined by qPCR. (B) Infectivity of AAV6-CMV-hPLAP as determined by the HT1080 cellular transduction assay (shown for 0, 5 and 60 minutes post-infusion).
Assessment of a 5-minute pre-circulation with Pluronic F-68 and the ability to prevent vector adsorbtion to the dialysis circuit.
(A) The HT1080 cell transduction assay as shown reveals a limited capacity to prevent vector binding to surface components within the circuit. (Pre indicates that the sample was drawn from a point in the circuit prior to the dialyzer and post indicates that the sample was taken after the dialyzer).
A 5% albumin priming step considerably prevents vector binding to the extracorporeal circuit.
(A) Vector genome analysis as determined by qPCR following a 5 minute priming course with 5% albumin. (B) Vector transduction capacity as revealed by the HT1080 assay with 5 minute albumin priming. (C) A 60 minute 5% albumin priming step further enhances vector recovery from the extracorporeal circuit as shown by qPCR vector genome analysis.
Whole blood pre-circulation of 30-minutes prevents vector absorption to the surface components of the extracorporeal circuit.
(A) Cesium chloride density gradient separating genome containing virions (Fulls) from virions that lack viral genomes (upper band). (B) Vector genome quantification as assessed by qPCR for the respective times. (C) HT1080 transduction assay evaluated at time 0, 30 and 60 minutes after vector infusion.
Oxygen was delivered at varying rates to the empty dialysate compartment of the gas exchanging dialyzer during the experiments that utilized whole blood with the inclusion of heparin. Blood gas measurements were taken from sample ports in the circuit before and after each dialyzer and the averages of values taken from a point in the circuit before the gas exchanging dialyzer and after the gas exchanging dialyzer were calculated. There was a dramatic increase in blood pO2 and expected decrease in pCO2 across the gas exchanging dialyzer ( Figure 6 , Table 1 ).
Whole blood pre-circulation time of 30-minutes with the incorporation of dialysis membrane for oxygen delivery.
(A) Image of system with oxygen being delivered across the lower membrane. (B) Evaluation of vector genomes retained over time as determined by southern blot quantification. (C) Partial pressure of oxygen over time (10 minutes, 1 L/min; 0-draw, time-0 from blood tube; 0-circ, time-0 from circuit; pre, arterial port; post, venous port).
Discussion
In this circulatory model ( Figure 1 ), a major limitation is vector loss due to non-specific binding, likely primarily contributed by surface charge topology of the vector, to artificial surfaces in the extracorporeal circuit. To address this problem we undertook a series of experiments to test AAV retention and transduction after the use of various circuit priming solutions. Our model utilized rAAV6 due to its ease of purification, ability to readily transduce a broad range of tissues and high degree of sequence identity to rAAV1 (6 amino acid dissimilarity), presently being utilized in the first European Commission approved marketing authorization for gene therapy. When priming with normal saline alone, vector loss occurred early on in the time course of the procedure, indicating that vector binding to the artificial surfaces of the extracorporeal circuit occurred relatively rapidly. Pluronic F-68 had previously been reported to limit non-specific binding of rAAV to artificial surfaces24. However, we did not observe an increase in vector recovery ( Figure 3 ). The reason Pluronic F-68 was ineffective in our experiment is unclear. In that Bennicelli et al reported a larger recovery of vector (albeit with rAAV2) we cannot rule out that there could be a dose effect given the concentration of vector used in our circuit was comparatively 1-log lower. Given the multi-component nature of our system, it is also possible that the dialysis membrane itself is not effectively blocked with P188 relative to the silicone or plastic components of the circuit. This would be supported by the observation of significantly less transduction being obtained from the venous sample port (post-dialyzer) relative to the arterial sample port (pre-dialyzer) at the 5-minute time point. It is also possible that some of the Pluronic F-68 may have passed through the dialysis membrane during the priming procedure leading to Pluronic F-68 loss from the circuit prior to the addition of rAAV. Pluronic F-68 has a molecular weight of 8400 daltons and therefore is small enough to pass through the pores on our high-flux dialyzer. Albumin was effective in preventing vector loss, presumably by coating the artificial surfaces and preventing rAAV6 non-specific binding. The efficacy of albumin in prevention of rAAV6 loss was improved with longer (60 min) dwell times prior to the addition of vector to the circuit.
The priming solution used for in vivo situations is likely to contain blood from the vasculature of the target tissue. To test the efficiency of rAAV6 recovery in the presence of blood we utilized non-human primate blood from a subject that was pre-screened for an excessively low titer (1:20) towards the neutralization of rAAV6 as determined by HT1080 inhibitory assays25. Here we utilized a rAAV6 vector preparation with the inclusion of a cesium chloride density centrifugation gradient ( Figure 5A ). In this manner, by eliminating virions that do not contain genomes, more subtle decreases in vector due to inhibition are more likely to be observed. Coincidentally, priming with blood was highly effective at preventing rAAV6 binding to the circuit components as well as preserving transduction capacity with minimal loss throughout the circulation procedure ( Figure 5B, 5C ).
The results of the chemistry analysis indicate that the partial pressure of oxygen can be increased and the partial pressure of carbon dioxide decreased using our technique to deliver oxygen to the blood in the circuit through a hollow fiber dialyzer ( Figure 6 , Table 1 ). The addition of oxygen to the circuit may allow longer periods of time for tissues to be isolated from the systemic circulation, thereby allowing more time for vector transduction to occur without incurring tissue ischemia and cellular injury due to hypoxia. The addition of oxygen to the extracorporeal circuit in this simple fashion is novel and even in the absence of gene therapy has the potential to be beneficial in many clinical settings.
Localized delivery of rAAV has the potential to allow for higher levels of transduction within target tissues through multiple mechanisms. Firstly, continuous recirculation through the vasculature provides for repeated and prolonged exposure of the vector to target tissues. The rAAV vector is too large (particle size ~22 nm, molecular weight estimated to be 3,900 KDa) to pass through a traditional dialysis membrane and, therefore, virus that has not been taken up by the cells during initial infusion can be drawn back into the circuit and delivered again to the target tissue in a continuous fashion, providing increased tissue exposure and increased efficiency of drug utilization. Secondly, pressure in the vasculature can be monitored during the procedure and, therefore, Starlings forces across the capillary wall can be estimated and modified to achieve optimal vector extravasation into the interstitial space for uptake by cells. Further, the dialysis equipment provides us with the capability to ultrafilter the circulatory fluid leading to removal of excess plasma water thus increasing vector concentration during the procedure. Lastly, performing diffusion dialysis and providing oxygen during the circulation procedure will allow control of the metabolic environment within the vasculature of isolated, anaerobic tissues.
Previous attempts at systemic intravenous delivery of rAAV for the treatment of genetic diseases in humans have been modestly successful but hampered by the development of unacceptable side effects such as systemic inflammatory response26, acute liver injury27,28 and difficulties in achieving vector cell transduction and stable expression of the transgene29,30. Targeted tissue delivery of rAAV has the potential benefit of reduction in systemic inflammatory response by confining the rAAV dose to diseased tissue and avoiding exposure of normal tissue to rAAV. In this model, diseased tissue can be isolated through surgical or interventional techniques and connected to an artificial circuit that can efficiently deliver and circulate rAAV over a period of time sufficient to allow greater rAAV transduction to the disease tissue while minimizing unwarranted exposure to non-target tissues.
In summary, the extracorporeal circuit for delivery of rAAV described in this manuscript has the potential for enhanced delivery of genetic material through efficient delivery of rAAV to disease tissues while minimizing potential side effects to the host. Future work will include testing of this concept in the isolated limb of large animal models under a variety of conditions such as circulation time, rate, varied pressure and transient higher and lower pH changes all in the presence of oxygenation.
Methods
Vector production
Recombinant AAV6 vector was prepared as previously described8,31. Briefly, genome-containing vectors were produced by CaPO4 transfection of HEK 293 cells with plasmids containing pDGM6 packaging/helper genes and rAAV-CMV-hPLAP-SV40pA vector genomes32. Cells were collected and processed through a microfluidizer (Microfluidics, Newton, MA) and vector particles were purified on a HiTrap heparin column (GE Healthcare, Chalfont St. Giles, UK). The virus was layered on a sucrose gradient (40%), spun at 27,000 rpm for 18 hours at 4°C and resolubilized in Hanks balanced salt solution (HBSS). Empty capsids were removed from the preparation by twice performing a CsCl gradient purification (preparation spun in an ultracentrifuge tube of 1.37 g/mL CsCl, 35 k r.p.m. for 24 hours at 6°C in an SW55ti), followed by dialysis into Hank's buffered salt solution (Invitrogen, Carlsbad, CA). Vector genome titer was determined by Southern blot with a DNA standard of known quantity.
Vector genome quantitation
Total nucleic acid from circulated samples was obtained by digesting vector samples with proteinase K followed by phenol chloroform extraction and ethanol precipitation. All real-time, quantitative PCRs were performed on a ABI 7500 Real Time PCR System (Applied Biosystems, Foster City, CA) in a total volume of 25 μl, consisting of 5 μl sample DNA, 12.5 μl TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA), 0.2 μM of each primer and 0.1 μM TaqMan custom probe (Applied Biosystems, Foster City, CA). Reaction conditions were 50°C for 2 minutes, 95°C for 10 minutes and 40 cycles of [95°C for 15 seconds followed by 60°C for 1 minute]. Each sample was analyzed in triplicate for concentration of total vector genome detection, the probe and primer set was targeted to the SV40 polyadenylation region of the vector genome. SV40 Primers: 5′-TTTTCACTGCATTCTAGTTGTGGTT-3′, 5′-ATCTCGACCTCGACTAGAGCATG-3′, TaqMan Probe: 5′-6FAM-ACTCATCAATGTATCT TATCATG-MBGNQ-3′. The AAV-CMV-hPLAP-SV40 plasmid was used as standard in order to obtain absolute genome copy numbers obtained for each sample. Southern blot analysis was additionally performed in order to confirm results of the qPCR on select experiments. In blood containing samples we utilized a 30% sucrose cushion in order to pellet a 0.1 ml sample containing the rAAV vector. Following removal of the cushion, the virus was then resuspended in loading buffer, boiled for 5 minutes, prior to loading onto the agarose gel. Samples were transferred to nylon membrane and hybridized utilizing a SV40 oligo probe labeled with phosphorus-32, exposed overnight and quantified using the phosphoimager (Storm 860, GE Healthcare, Uppsala Sweden).
In vitro transduction assays
HT1080 cells were seeded in 12-well culture plates, at 9,000 cells/well in Dulbecco's Modified Eagle Medium (Gibco, Carlsbad, CA) containing 10% FBS (HyClone, Logan, UT), 1% L-glutamine, 1% penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO). Input vector or aliquots from the extracorporeal circuit were diluted in HBSS (Invitrogen, Carlsbad, CA) to total volume of 0.2 ml being represented as an equivalent ratio to one another. The dilutions were then added to HT1080 cultures 24 hours after seeding the wells. Cells were incubated at 37°C, 5% CO2 for 3 days. Media was removed and cells were fixed in 3.7% formaldehyde in phosphate buffered saline (PBS) for 10 minutes. Cells were washed 3× in PBS and endogenous AP was inactivated by heating cells for 1 hour at 65°C, in PBS. Cells were then washed with room temperature PBS and Sigma FAST BCIP/NBT substrate solution (Sigma, St. Louis, MO) was applied. Cells were stained in the dark at room temperature for 70 minutes, rinsed 2× in PBS and finally set in water. Human placental alkaline phosphatase (hPLAP) positive cells were imaged by light microscopy (Olympus SZX16).
Blood gas & electrolyte analysis
Measurements of blood gases and electrolytes were performed prior to loading the extracorporeal circuit and at time increments throughout with a blood gas analyzer (Abaxis iSTAT1, CG8+ cartridges).
Circuit design
The circuit consists of low volume hemodialysis blood tubing (Combiset, Fresenius) with a priming volume of 97 mL. The tubing is composed of polyvinyl chloride (PVC) and is sterilized with ethylene oxide. The tubing is connected in series with two hemodialyzers. The dialysis filters used include a low-flux, hollow fiber, single use dialyzer (F3, Fresenius) and high-flux, hollow-fiber, single use dialyzer (6H, Gambro). The F3 membrane is composed of polysulfone with a fiber inner diameter of 200 μm, membrane wall thickness of 40 μm and a surface area of 0.4 m2. The priming volume is 24 mL, ultrafiltration coefficient 1.7 mL/mmHg/min. The housing of the dialyzer is composed of polycarbonate, potting material is polyurethane and the dialyzer is sterilized with ethylene oxide. The 6H membrane is composed of a polyamide mix with a fiber inner diameter 215 μm, membrane wall thickness of 50 μm and a surface area of 0.6 m2. The priming volume is 52 mL, ultrafiltration coefficient 33 mL/mmHg/min. The housing of the dialyzer is composed of polycarbonate, the potting material composed of polyurethane and the dialyzer is sterilized with steam. Following the initial dialysis filter, a second filter is placed in series to serve as the point of oxygen delivery. The dialysate compartment of this filter is connected to a oxygen tank which allows oxygen to flow by the membrane and diffuse into the circuit. The circuit tubing and dialyzers are attached to a hemodialysis machine (Fresenius, 2008H) which consists of a roller pump to control movement of circulatory fluid through the circuit, pressure monitors, a dialysate proportioning system for formation of dialysate and various other safety monitors. Sampling ports are available in the circuit for measures of interest such as vector concentration. The products utilized in this project are all FDA approved for use in humans on hemodialysis, are generally accepted as safe and are composed of the most biocompatible materials available today.
Circuit operation
Prior to each in vitro procedure the tubing and dialyzer were primed with a solution of lactated ringers or normal saline. After the air was voided from the tubing the arterial and venous limbs of the circuit were connected to form a continuous loop. The priming solution was then allowed to circulate for a period of 10–15 minutes with dialysis occurring, leading to the formation of a uniform physiologic solution in the circuit with pH and electrolyte composition similar to the dialysate. After the pre-procedure circulation period, addition of a coating solution such as albumin (BSA) or surfactant (P188) was administered to the circuit. The solution was allowed to circulate or dwell for a period of time prior to the addition of vector. After the setup and priming procedure was complete, vector in a volume of 1 ml of Hanks buffered saline (~1 × 1012 total vector genomes) was added to the circuit and allowed to circulate. Flow of the circulating fluid was kept between 150–200 ml/min. Dialysate flow was kept at 500 ml/min. Final dialysate was composed of 2 mEq/L of KCl, 35 mEq/L of bicarbonate, 133 mEq/L of sodium, 2.5 mEq/L of calcium, 1 mEq/L of magnesium, 8 mEq/L of acetate, 101 mEq/L of chloride and 100 mg/dL of dextrose. The duration of circulation was 60 minutes, samples were taken for analysis at time 0, 5 min, 10 min and then every 10 min for the duration of the procedure.
References
Atchison, R. W., Casto, B. C. & Hammon, W. M. Adenovirus-Associated Defective Virus Particles. Science 149, 754–6 (1965).
Muzyczka, N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol 158, 97–129 (1992).
Muzyczka, N. & Berns, K. I. Parvoviridae: The viruses and their replication. In: Knipe, D. M., Howley, P. M. (eds). Fundamental Virology 4th edn. Lippincott, Williams and Wilkens: Philadelphia, pp 1089–1122 (2001).
Rutledge, E. A., Halbert, C. L. & Russell, D. W. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J Virol 72 (1), 309–19 (1998).
Xiao, W., Chirmule, N., Berta, S. C., McCullough, B., Gao, G. & Wilson, J. M. Gene therapy vectors based on adeno-associated virus type 1. Journal of Virology 73 (5), 3994–4003 (1999).
Gao, G. P., Alvira, M. R., Wang, L., Calcedo, R., Johnston, J. & Wilson, J. M. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proceedings of the National Academy of Sciences of the United States of America 99 (18), 11854–9 (2002).
Chao, H., Liu, Y., Rabinowitz, J., Li, C., Samulski, R. J. & Walsh, C. E. Several Log Increase in Therapeutic Transgene Delivery by Distinct Adeno-Associated Viral Serotype Vectors. Molecular Therapy 2 (6), 619–623 (2000).
Halbert, C. L., Allen, J. M. & Miller, A. D. Adeno-associated virus type 6 (AAV6) vectors mediate efficient transduction of airway epithelial cells in mouse lungs compared to that of AAV2 vectors. Journal of Virology 75 (14), 6615–24 (2001).
Duan, D., Yan, Z., Yue, Y., Ding, W. & Engelhardt, J. F. Enhancement of muscle gene delivery with pseudotyped adeno-associated virus type 5 correlates with myoblast differentiation. Journal of Virology 75 (16), 7662–71 (2001).
Grimm, D., Kay, M. A. & Kleinschmidt, J. A. Helper virus-free, optically controllable and two-plasmid-based production of adeno-associated virus vectors of serotypes 1 to 6. Molecular Therapy 7 (6), 839–50 (2003).
Rabinowitz, J. E. & Samulski, J. Adeno-associated virus expression systems for gene transfer. Curr Opin Biotechnol 9 (5), 470–5 (1998).
Schultz, B. R. & Chamberlain, J. S. Recombinant adeno-associated virus transduction and integration. Mol Ther 16 (7), 1189–99 (2008).
Xiao, X., Li, J. & Samulski, R. J. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. Journal of Virology 70 (11), 8098–108 (1996).
Fisher, K. J., Jooss, K., Alston, J., Yang, Y., Haecker, S. E. & High, K. et al. Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med 3 (3), 306–12 (1997).
Song, S., Morgan, M., Ellis, T., Poirier, A., Chesnut, K. & Wang, J. et al. Sustained secretion of human alpha-1-antitrypsin from murine muscle transduced with adeno-associated virus vectors. Proceedings of the National Academy of Sciences of the United States of America 95 (24), 14384–8 (1998).
Herzog, R. W., Yang, E. Y., Couto, L. B., Hagstrom, J. N., Elwell, D. & Fields, P. A. et al. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med 5 (1), 56–63 (1999).
Manno, C. S., Chew, A. J., Hutchison, S., Larson, P. J., Herzog, R. W. & Arruda, V. R. et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood 101 (8), 2963–2972 (2003).
Favaro, P., Finn, J. D., Siner, J. I., Wright, J. F., High, K. A. & Arruda, V. R. Safety of liver gene transfer following peripheral intravascular delivery of adeno-associated virus (AAV)-5 and AAV-6 in a large animal model. Hum Gene Ther 22 (7), 843–52 (2011).
Wang, L., Calcedo, R., Bell, P., Lin, J., Grant, R. L. & Siegel, D. L. et al. Impact of pre-existing immunity on gene transfer to nonhuman primate liver with adeno-associated virus 8 vectors. Hum Gene Ther 22 (11), 1389–401 (2011).
Breous, E., Somanathan, S., Bell, P. & Wilson, J. M. Inflammation promotes the loss of adeno-associated virus-mediated transgene expression in mouse liver. Gastroenterology 141 (1), 348–57, 357 e1–3 (2011).
Goldman, M., Lagmiche, M., Dhaene, M., Amraoui, Z., Thayse, C. & Vanherweghem, J. L. Adsorption of beta 2-microglobulin on dialysis membranes: comparison of different dialyzers and effects of reuse procedures. Int J Artif Organs 12 (6), 373–8 (1989).
Urbani, A., Sirolli, V., Lupisella, S., Levi-Mortera, S., Pavone, B. & Pieroni, L. et al. Proteomic investigations on the effect of different membrane materials on blood protein adsorption during haemodialysis. Blood Transfus 10 Suppl 2, s101–12 (2012).
Urbani, A., Lupisella, S., Sirolli, V., Bucci, S., Amoroso, L. & Pavone, B. et al. Proteomic analysis of protein adsorption capacity of different haemodialysis membranes. Mol Biosyst 8 (4), 1029–39 (2012).
Bennicelli, J., Wright, J. F., Komaromy, A., Jacobs, J. B., Hauck, B. & Zelenaia, O. et al. Reversal of blindness in animal models of leber congenital amaurosis using optimized AAV2-mediated gene transfer. Mol Ther 16 (3), 458–65 (2008).
Halbert, C. L., Standaert, T. A., Wilson, C. B. & Miller, A. D. Successful readministration of adeno-associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure. J Virol 72 (12), 9795–805 (1998).
Raper, S. E., Chirmule, N., Lee, F. S., Wivel, N. A., Bagg, A. & Gao, G. P. et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab 80 (1–2), 148–58 (2003).
Nathwani, A. C., Tuddenham, E. G., Rangarajan, S., Rosales, C., McIntosh, J. & Linch, D. C. et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 365 (25), 2357–65 (2011).
Raper, S. E., Yudkoff, M., Chirmule, N., Gao, G. P., Nunes, F. & Haskal, Z. J. et al. A pilot study of in vivo liver-directed gene transfer with an adenoviral vector in partial ornithine transcarbamylase deficiency. Hum Gene Ther 13 (1), 163–75 (2002).
Manno, C. S., Pierce, G. F., Arruda, V. R., Glader, B., Ragni, M. & Rasko, J. J. et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med 12 (3), 342–7 (2006).
Mingozzi, F., Maus, M. V., Hui, D. J., Sabatino, D. E., Murphy, S. L. & Rasko, J. E. et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med 13 (4), 419–22 (2007).
Blankinship, M. J., Gregorevic, P., Allen, J. M., Harper, S. Q., Harper, H. & Halbert, C. L. et al. Efficient transduction of skeletal muscle using vectors based on adeno-associated virus serotype 6. Molecular Therapy 10 (4), 671–8 (2004).
Gregorevic, P., Blankinship, M. J., Allen, J. M., Crawford, R. W., Meuse, L. & Miller, D. G. et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med 10 (8), 828–34 (2004).
Acknowledgements
We thank James Allen for critical reading of the manuscript and Steve Hauschka for insightful experimental suggestions. Additionally we thank Arlene Chen for administrative support. SB was supported by the Advanced Nephrology Dialysis Fellowship at the University of Washington with funding from the Northwest Kidney Centers. JSC supported by funding from the National Institutes of Health (grants AR40864 and AG033610). The authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Contributions
S.B. and G.L.O. wrote the main manuscript text and prepared all figures. E.F. facilitated rAAV vector production and purification, while S.A., J.S.C. and J.B.H. provided unique insight toward experimental design. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Bieber, S., Halldorson, J., Finn, E. et al. Extracorporeal Delivery of rAAV with Metabolic Exchange and Oxygenation. Sci Rep 3, 1538 (2013). https://doi.org/10.1038/srep01538
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep01538
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.