Abstract
Antiphospholipid syndrome is associated with an increased risk of thrombosis and pregnancy loss. Annexin A5 (Anxa5) is a candidate autoantigen. It is not known, however, whether endogenous Anxa5 prevents foetal loss during normal pregnancy. We found significant reductions in litter size and foetal weight in Anxa5-null mice (Anxa5-KO). These changes occurred even when only the mother was Anxa5-KO. A small amount of placental fibrin deposition was observed in the decidual tissues, but did not noticeably differ between wild-type and Anxa5-KO mice. However, immunoreactivity for integrin beta 3/CD61, a platelet marker, was demonstrated within thrombi in the arterial canals only in Anxa5-KO mothers. Subcutaneous administration of the anticoagulant heparin to pregnant Anxa5-KO mice significantly reduced pregnancy loss, suggesting that maternal Anxa5 is crucial for maintaining intact placental circulation. Hence, the presence of maternal Anxa5 minimises the risk of thrombosis in the placental circulation and reduces the risk of foetal loss.
Similar content being viewed by others
Introduction
Pregnancy loss is not a rare complication in human pregnancy. Anti-phospholipid syndrome is thought to be a major cause of early pregnancy loss1. This condition is characterised by the presence of anti-phospholipid antibody. In fact, auto-antibodies to various phospholipids and phospholipid binding proteins have been reported2. Anxa5 has been proposed to be a common auto-antigen in anti-phospholipid syndrome3.
Anxa5 is a member of the annexin family of proteins, which consists of 12 structurally related, highly conserved proteins in humans and mice4. Anxa5 was originally discovered as a candidate anticoagulant protein in the placenta5,6, but its involvement in the prevention of inappropriate coagulation in the placenta has not been elucidated. In the human and mouse placenta, Anxa5 is extensively distributed on the cell surface of syncytiotrophoblasts7,8,9. Patients with antiphospholipid syndrome and lupus erythematosus often exhibit autoantibodies against Anxa5 and pregnant patients sometimes show spontaneous foetal loss during the early stages of pregnancy10,11,12. However, there has been no direct in vivo evidence that endogenous Anxa5, when expressed by either the mother or the foetus, prevents foetal loss during pregnancy.
The annexins are characterised by their related structures, which are composed of four repeats (eight for annexin A6) of approximately 60 amino acids13 that allow calcium-dependent binding to phospholipid membranes. Anxa5 has been shown to be involved in multiple cellular processes, such as intracellular signalling, mineralisation of cartilage and inhibition of phospholipase A2 and protein kinase C14,15,16,17,18. Anxa5 is well known for its capacity to detect early apoptotic cells due to its high affinity for exposed phosphatidylserine on the surfaces of these cells19. Therefore, it has been proposed that the binding of Anxa5 to cell surface-exposed phosphatidylserine on vascular endothelial cells in the placenta is crucial for suppressing inappropriate blood coagulation during pregnancy3,11,20. Binding of autoantibodies to Anxa5 can disrupt the protective shield in patients with antiphospholipid syndrome, causing placental thrombosis and, ultimately, pregnancy loss3,8,11,21. Although, in support of this view, intravenous administration of antibodies against Anxa5 to pregnant mice has been shown to lead to placental thrombosis and foetal loss8, it is not clear whether this is a nonspecific reaction to acutely formed abundant antigen-antibody complexes in the circulation.
We established an Anxa5-null mouse model (Anxa5-KO) and our initial studies showed that the strain was viable and fertile and lacked an obviously altered phenotype22. In the present study, we demonstrate that the number of foetuses and hence the litter size, were significantly reduced by deficient maternal Anxa5 production. This result reveals that the maternal supply of Anxa5 to the circulation is necessary for maintaining a fully intact pregnancy.
Results
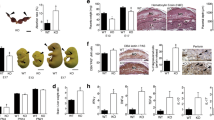
The litter sizes of Anxa5-KO derived from Anxa5-KO x Anxa5-KO crosses were significantly smaller than the sizes of litters from C57BL/6J (WT) x WT crosses (Fig. 1-a, Anxa5-KO: 6.30 ± 0.35 vs. WT: 8.33 ± 0.30, n = 30, p<0.001). When the number of ovulated ova on the morning of oestrus was counted by dissecting the oviduct and examining the ova in the ampulla, no difference was found in the number of ova in the Anxa5-KO and WT mice (Anxa5-KO: 8.28 ± 0.42 vs. WT: 8.86 ± 0.80, n = 7). Therefore, some embryos were lost during pregnancy in the Anxa5-KO mice. By analysing the progression of pregnancy, a clear reduction in the average litter size was observed between days 12 and 18. There was no difference between the number of embryos on day 18 and the newborn litter size (Fig. 1-a). Foetal loss in the Anxa5-KO was consistently observed regardless of parity (parity n = 1 to 3, data not shown).
The deficiency of maternal Anxa5 affects embryonic growth and litter size.
a: Number of ovulated ova, embryos and litter size in Anxa5-KO (AX5−/−) mice. The number of ova in the oviduct was counted on the morning of oestrus for Anxa5-KO and C57BL/6J (WT) mice. The number of embryos was counted on each pregnancy day (12, 15 and 18). The litter size was counted on the day of delivery. The values are expressed as the mean and the standard error of the mean. Asterisks reveal a significant difference (Bonferroni method p<0.05). b: The conceptuses were observed on pregnancy days 9, 12, 15 and 18 of WT and Anxa5-KO mice. The arrows indicate smaller foetuses. (Scale bar = 1 cm). c: A representative picture of an embryo and placenta on pregnancy day 15 corresponding to samples WT #3 and Anxa5-KO #2 in supplemental table 1. d: The weight of the embryos on pregnancy day 15. Foetal weight was measured on pregnancy day 15 (n = 48 for WT, n = 46 for Anxa5-KO, individual data are presented in supplemental table 1).
Smaller foetuses were found in the uteruses of Anxa5-KO mice on days 12, 15 and 18 of pregnancy (Fig. 1-b). Foetuses of WT and Anxa5-KO mice were compared on pregnancy day 15 (Fig. 1-c, d). Both the number (WT: 9.6 ± 0.24, n = 5 vs. Anxa5-KO: 6.5 ± 0.48, n = 7) and weight (Fig. 1-d) of the foetuses were smaller in Anxa5-KO mice (Supplemental table 1). In addition to living, intact foetuses, the existence of remnants of the conceptus in six of seven pregnant Anxa5-KO mice was observed on day 15 of pregnancy (Supplemental table 1).
There was no difference in the plasma levels of progesterone on days 12 and 18 of pregnancy between the Anxa5-KO and WT mice (day 12: 115.9 ± 16.5 vs. 126.7 ± 19.7 ng/ml, respectively, n = 5, p>0.05; day 18: 107.4 ± 25.3 vs. 68.5 ± 11.5 ng/ml, respectively, n = 5, p>0.05). In addition, the lengths of pregnancy (Anxa5-KO: 20.5 ± 0.5, n = 6 vs. WT: 21 ± 0.8 days, n = 8) and the oestrous cycle (Anxa5-KO: 5.2 ± 0.2, n = 16 vs. WT: 5.2 ± 0.2 days, n = 25) showed no significant differences (p>0.05 respectively).
The placentae on day 15 of pregnancy showed fewer erythrocytes in both the labyrinth and junctional zones of Anxa5-KO mice (Fig. 2-a, b). There were many foetal erythrocytes (large eosinophylic cells with nucleus) but fewer maternal erythrocytes (small and without nucleus) in Anxa5-KO placenta (Fig. 2c, d). It was revealed that only nucleated foetal erythrocytes were present in the tissue of absorbed conceptuses (Fig. 2-e). Large pink homogeneous structures, which were likely thrombi, were observed both in the labyrinth and junctional zone of the placentae of smaller foetuses on day 18 (Fig. 2-f).
Histological analysis of the placenta.
The placentas were collected on pregnancy days 15 and 18. They were stained with hematoxylin-eosin. a, b: lower magnification. c, d: higher magnification of labyrinth zone. e: Conceptus tissue being absorbed. f: The placenta of a growth-retarded foetus collected on pregnancy day 18. The yellow and green arrows indicate the labyrinth zone and the junctional zone, respectively. The black and white arrows indicate homogeneous undefined extracellular matrix material in each placenta.
Immunohistochemistry with anti-integrin beta 3/CD61, a platelet marker, clearly showed thrombi in the placentae of Anxa5-KO mice (Fig. 3). No such thrombus was observed in the WT placentae. Thrombi were observed in all placentae of healthy conceptuses in the Anxa5-KO mice (ten placentae from three mice). Thrombi were observed beneath the trophoblast giant cell layer. Alternatively, fibrin deposition detected with immunohistochemistry for fibrinogen/fibrin interactions were mainly in the decidual layer, outside the trophoblast giant cell layer, of WT and Anxa5-KO mice (Supplemental figure 1). The intensity of fibrin immunoreactivity was not different between the WT and Anxa5-KO mice.
Cross-breeding experiments of Anxa5-KO and WT mice showed that only litters bred using Anxa5-KO females (f) showed a reduced number of pups (Fig. 4-a). Crossing Anxa5-KO females (f) with Anxa5-KO males (m) or with WT (m) resulted in the same reduction in litter size (Fig. 4-a). Immunohistochemistry with the placentae collected from four different combinations of parents in the cross-breeding experiments on pregnancy day 15 clearly showed that the “wire loop” pattern of Anxa5-coated syncytiotrophoblast surfaces and vessel walls was lost in Anxa5-KO mothers (Fig. 4-b, Anxa5-KO (f)).
Cross-breeding of Anxa5-KO and WT.
a: Changes in the litter size after cross breeding of Anxa5-KO mice and WT mice. WT and Anxa5-KO (AX5−/−) were mated in different combinations. Asterisks denote significant differences (Bonferroni test p<0.05). b: Immunohistochemical distribution of Anxa5 in the placentae of mice on pregnancy day 15. WT and Anxa5-KO were mated in different combinations. WT (f): the mother was WT. WT (m): the father was WT. Anxa5-KO (f): the mother was Anxa5-KO. Anxa5-KO (m): the father was Anxa5-KO. The arrows indicate that there was no Anxa5 on the surface of the syncytiotrophoblasts in the labyrinth zone (black) or the walls of the arterial canal of the junctional zone (white) of the placenta from the combination of Anxa5-KO (f) and WT (m).
Blood taken from Anxa5-KO mice coagulated faster than that from WT mice, demonstrating lower levels of anticoagulant activity in Anxa5-KO mice (Fig. 5-a). Finally, we found that the administration of heparin (100 IU/day on pregnancy days 12, 14 and 16) to Anxa5-KO mothers significantly increased the litter size (Fig. 5-b).
The deficiency of maternal Anxa5 affects blood coagulation.
a: The blood clotting time of Anxa5-KO. Blood (500 μl) was obtained by heart puncture and warmed at 37°C. The clotting time was measured by shaking the tube every 30 seconds. The values are expressed as the mean and the standard error of the mean. The Mann-Whitney's U-test was performed. b: The effect of heparin on the litter size of Anxa5-KO mice. The Anxa5-KO mice were subcutaneously administered saline or heparin (100 IU) on pregnancy days 12, 14 and 16. The Student's t-test was performed (*p<0.05).
Discussion
The results of the present study clearly demonstrate that the deficiency of Anxa5 results in significant foetal loss. As smaller foetuses were found in the uteruses of Anxa5-KO mothers, embryos were restricted in growth. Severely growth-restricted foetuses may have died later and been absorbed, resulting in the reduction of litter size in Anxa5-KO mice. The inappropriate formation of thrombi in the placental circulation is linked to foetal growth retardation and absorption. These features of pregnant Anxa5-KO mice are similar to complications of infertility caused by anti-phospholipid syndrome1,2,3,23,24.
Anxa5 has been postulated to be a significant auto-antigen for pregnancy loss3,21,25. The present results provide direct evidence that maternal Anxa5 acts as an anti-thrombotic agent during pregnancy. Foetuses are lost only when maternal Anxa5 is absent. Therefore, the absence of Anxa5, or possibly mutations affecting its binding capacity to phospholipids, can explain the molecular mechanism of pregnancy loss in patients suffering from antiphospholipid syndrome and lupus erythematosus3,26,27. The placentae of Anxa5-KO were always anaemic and thrombi were also found even healthy placentae. These changes were thought to cause smaller embryos and increase the likelihood of foetal death. Fibrin deposition was mainly observed in the decidual tissues of both WT and Anxa5-KO mice, but thrombi were detected in the placentae only of Anxa5-KO placenta. This finding indicates that platelet activation is primarily induced under Anxa5-defiencnt conditions not depending on the quantity of fibrin deposition. As some other annexins were also shown to have an affinity for phosphatidyl serine28, it is possible that they would compensate somewhat the deficiency of Anxa5. Our data suggest that Anxa5 could play a role in the prevention of inappropriate platelet activation. Placental Anxa5 is necessary for reducing the possibility of inappropriate thrombosis and for a fully intact pregnancy.
Additionally, there was no difference in the plasma levels of progesterone, the length of pregnancy or the length of the oestrous cycle, indicating the intact reproductive function of Anxa5-KO mice with the exception of their tendency to form thrombi in their placentae.
Cross-breeding experiments of Anxa5-KO and WT mice showed that the presence of maternal Anxa5 is crucial for the maintenance of pregnancy. Only litters bred using Anxa5-KO (f) showed a reduced number of pups. Hence, heterozygous embryos delivered from Anxa5-KO mice did not rescue the phenotype. Anxa5 was shown to be bound to the surface of the syncytiotrophoblasts of placental vessels, potentially shielding the phosphatidylserine signal on the outer cell surface7. Immunohistochemistry of the placentae showed that the Anxa5-coating of the walls was lost in Anxa5-KO mothers. These data clearly indicate that Anxa5 is supplied maternally, although the placenta is entirely of foetal origin29. Furthermore, blood from Anxa5-KO mice showed lower levels of anticoagulant activity. This observation, in concert with the finding that the formation of thrombi in the arterial circuitry did not follow fibrin deposition, suggests that it is also important for platelets to be shielded with Anxa530. Finally, we found that the administration of heparin to Anxa5-KO mothers significantly increased litter size. Heparin inhibits thrombin and thrombin activates platelets31. So, the administration of Anxa5 is thought to inhibit platelet thrombi formation. These data clearly demonstrate that foetal loss in Anxa5-KO mice is caused by inappropriate thrombogenesis.
The mouse placenta is hemochorial and structures functionally equivalent to villi in the human placenta are directly bathed in the mother's blood, as in humans29,32. Thus, the present results obtained from this Anxa5-deficient mouse model could be extrapolated to understand the pathogenesis of preeclampsia and infertility caused by antiphospholipid syndrome in humans.
Methods
Animals
The Anxa5-KO line was first established with a mixed 129Sv/C57BL/6 background and then backcrossed to the C57BL/6 background for more than 10 generations22. Wild-type C57BL/6J mice (WT) were obtained from CLEA Japan, Inc. (Tokyo, Japan) for control experiments. The mice bred in our laboratory were maintained at 23 ± 3°C with a controlled light cycle of 14L:10D (light on 5:00–19:00 h). Food and tap water were supplied ad libitum. All experiments were performed according to the guidelines for animal experiments of Kitasato University and the experimental plans were approved by the Committee for Laboratory Animals, Care and Use of the School of Veterinary Medicine, Kitasato University.
Vaginal smears were taken daily and pregnancy was induced by placing a female into a male's cage on the evening of pro-oestrous. The presence of a vaginal plug was confirmed the next morning and the day of oestrous was designated as day 0 of pregnancy. The pups were counted on the morning of delivery. To count the ovulated ova, each mouse was lightly anesthetised with diethyl ether (Kanto Chemical Co., Inc., Tokyo, Japan) and sacrificed using cervical dislocation on the morning of oestrus. The oviduct was dissected and the number of ova in the oviduct was counted. Pregnant animals were also sacrificed on pregnancy days 12, 15 and 18 to count the foetuses in the uteruses of Anxa5-KO and WT mice.
Tissue preparation for histological analysis
The placentas were collected from mice in various experimental groups and fixed in 4% paraformaldehyde overnight at 4°C. The tissue blocks were washed, dehydrated and embedded in paraffin. Four-micron sections were made and dried in an incubator at 37°C overnight. The dried sections were stained with hematoxylin-eosin or subjected to immunohistochemistry for Anxa5, CD61 and fibrin. The hematoxylin and eosin solutions were obtained from Muto Pure Chemicals Co., LTD. (Tokyo, Japan).
Immunohistochemistry
After hydration, the sections were incubated with 5% normal rabbit serum for 1 hour to reduce non-specific antibody binding. The primary antibody was anti-Anxa5 rabbit serum raised in our laboratory33. Anti-integrin beta-3A/CD61 [EPR2417Y] monoclonal rabbit IgG from GeneTex (Funakoshi, Tokyo, Japan) and anti-fibrinogen gamma (G-20) goat serum (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.) were also used. These antibodies were used at a dilution of 1:100 and 1:1,000, respectively. The antiserum for Anxa5 was diluted to 1:10,000 and the sections were incubated with the serum overnight at 4°C. The second antibody system for visualisation was the ImmPRESS reagent anti-rabbit, anti-mouse or anti-goat IgG POD (Vector Laboratories, Burlingame, CA, U.S.A.). The specimens were counterstained with hematoxylin.
Measurement of blood clotting time
The mice were lightly anesthetised with diethyl ether. The blood samples (500 μl) were then obtained by cardiopuncture and placed immediately into plastic test tubes. The tubes were incubated at 37°C and shaken gently every 30 seconds to confirm coagulation. The time needed for complete coagulation was measured.
Administration of heparin
Heparin was obtained from Ajinomoto Co., Inc. (Tokyo, Japan). Heparin (100 IU/0.1 ml) was given subcutaneously in the backs of Anxa5-KO mice every other day from pregnancy day 12 to 18.
Time-resolved fluorometric assay of progesterone
The progesterone concentration was determined with a time-resolved fluorometric immunoassay using the Delfia system (Perkin Elmer Life Sciences, Tokyo, Japan). Anti-progesterone raised in our laboratory was labelled with Europium using the Delfia Eu-labeling kit (Perkin Elmer Life Sciences). An immunoplate (96 wells, Nunc Co., Roskilde, Denmark) was coated with 100 μl/well of progesterone-BSA (2.5 μg/ml, Steraloids Inc., Newport, RI) and blocked with 200 μl/well of a blocking buffer (50 mM Na2HPO4, 0.1% BSA). Then, an optimally diluted sample (100 μl/well) and Eu-labelled antibody (100 μl/well) were incubated for 2 hours at room temperature and the intensity of the bound label was measured using the Delfia Plate Fluorometer. All samples were run in duplicate.
Statistics
Each value represents the mean ± standard error of the mean. Statistical analysis was performed using the Student's t-test for the comparison of two groups and the Bonferroni test for the multiple comparison. The Mann-Whitney U-test was used for discontinuous values obtained in the blood clotting test. P values less than 0.05 were considered statistically significant.
References
Shetty, S. & Ghosh, K. Anti-phospholipid antibodies and other immunological causes of recurrent foetal loss--a review of literature of various therapeutic protocols. Am J Reprod Immunol 62, 9–24 (2009).
Blank, M. & Shoenfeld, Y. Antiphospholipid antibody-mediated reproductive failure in antiphospholipid syndrome. Clin Rev Allergy Immunol 38, 141–147 (2010).
Rand, J. H., Wu, X. X., Quinn, A. S. & Taatjes, D. J. The annexin A5-mediated pathogenic mechanism in the antiphospholipid syndrome: role in pregnancy losses and thrombosis. Lupus 19, 460–469 (2010).
Gerke, V. & Moss, S. E. Annexins: from structure to function. Physiol Rev 82, 331–371 (2002).
Iwasaki, A. et al. Structure and expression of cDNA for an inhibitor of blood coagulation isolated from human placenta: a new lipocortin-like protein. J Biochem 102, 1261–1273 (1987).
Funakoshi, T., Heimark, R. L., Hendrickson, L. E., McMullen, B. A. & Fujikawa, K. Human placental anticoagulant protein: isolation and characterization. Biochemistry 26, 5572–5578 (1987).
Krikun, G. et al. The expression of the placental anticoagulant protein, annexin V, by villous trophoblasts: immunolocalization and in vitro regulation. Placenta 15, 601–612 (1994).
Wang, X., Campos, B., Kaetzel, M. A. & Dedman, J. R. Annexin V is critical in the maintenance of murine placental integrity. Am J Obstet Gynecol 180, 1008–1016 (1999).
Brachvogel, B. et al. Perivascular cells expressing annexin A5 define a novel mesenchymal stem cell-like population with the capacity to differentiate into multiple mesenchymal lineages. Development 132, 2657–2668 (2005).
Gris, J. C. et al. Antiphospholipid and antiprotein syndromes in non-thrombotic, non-autoimmune women with unexplained recurrent primary early foetal loss. The Nimes Obstetricians and Haematologists Study--NOHA. Thromb Haemost 84, 228–236 (2000).
Matsubayashi, H. et al. Anti-annexin V antibodies in patients with early pregnancy loss or implantation failures. Fertil Steril 76, 694–699 (2001).
Gris, J. C. et al. Antiphospholipid/antiprotein antibodies, hemostasis-related autoantibodies and plasma homocysteine as risk factors for a first early pregnancy loss: a matched case-control study. Blood 102, 3504–3513 (2003).
Crompton, M. R., Moss, S. E. & Crumpton, M. J. Diversity in the lipocortin/calpactin family. Cell 55, 1–3 (1988).
Kawaminami, M. et al. Annexin 5 messenger ribonucleic acid expression in pituitary gonadotropes is induced by gonadotropin-releasing hormone (GnRH) and modulates GnRH stimulation of gonadotropin release. Neuroendocrinology 75, 2–11 (2002).
Kawaminami, M., Shibata, Y., Yaji, A., Kurusu, S. & Hashimoto, I. Prolactin inhibits annexin 5 expression and apoptosis in the corpus luteum of pseudopregnant rats: involvement of local gonadotropin-releasing hormone. Endocrinology 144, 3625–3631 (2003).
Kawaminami, M. et al. Gonadotropin-releasing hormone stimulates annexin 5 messenger ribonucleic acid expression in the anterior pituitary cells. Biochem Biophys Res Commun 291, 915–920 (2002).
Kim, H. J. & Kirsch, T. Collagen/annexin V interactions regulate chondrocyte mineralization. J Biol Chem 283, 10310–10317 (2008).
Moss, S. E. Annexins. Trends Cell Biol 7, 87–89 (1997).
Koopman, G. et al. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 84, 1415–1420 (1994).
Kaburaki, J., Kuwana, M., Yamamoto, M., Kawai, S. & Ikeda, Y. Clinical significance of anti-annexin V antibodies in patients with systemic lupus erythematosus. Am J Hematol 54, 209–213 (1997).
Alijotas-Reig, J. et al. Anti-annexin A5 antibodies in women with spontaneous pregnancy loss. Med Clin (Barc) 134, 433–438 (2010).
Brachvogel, B. et al. Annexin A5 is not essential for skeletal development. Mol Cell Biol 23, 2907–2913 (2003).
Abrahams, V. M. Mechanisms of antiphospholipid antibody-associated pregnancy complications. Thromb Res 124, 521–525 (2009).
Hradecky, L., Subrt, I. & Ulcova-Gallova, Z. Urgent termination of pregnancy in pre-eclampsia and panel of antiphospholipid antibodies. Am J Reprod Immunol 62, 412–417 (2009).
Hayes, M. J., Longbottom, R. E., Evans, M. A. & Moss, S. E. Annexinopathies. Subcell Biochem 45, 1–28 (2007).
Miyamura, H. et al. Polymorphisms in the annexin A5 gene promoter in Japanese women with recurrent pregnancy loss. Mol Hum Reprod 17, 447–452 (2011).
Markoff, A. et al. Reduced allele specific annexin A5 mRNA levels in placentas carrying the M2/ANXA5 allele. Placenta 31, 937–940 (2010).
Rosenbaum, S. et al. Identification of novel binding partners (annexins) for the cell death signal phosphatidylserine and definition of their recognition motif. J Biol Chem 286, 5708–5716 (2011).
Rossant, J. & Cross, J. C. Placental development: lessons from mouse mutants. Nat Rev Genet 2, 538–548 (2001).
Rand, M. L. et al. Diannexin, an annexin A5 homodimer, binds phosphatidylserine with high affinity and is a potent inhibitor of platelet-mediated events during thrombus formation. J Thromb Haemost 10, 1109–19 (2012).
De Candia, E. Mechanisms of platelet activation by thrombin: a short history. Thromb Res 129, 250–256 (2012).
Cross, J. C. et al. Trophoblast functions, angiogenesis and remodeling of the maternal vasculature in the placenta. Mol Cell Endocrinol 187, 207–212 (2002).
Kawaminami, M. et al. Immunocytochemical localization of annexin 5, a calcium-dependent phospholipid-binding protein, in rat endocrine organs. Cell Tissue Res 292, 85–89 (1998).
Acknowledgements
We thank Ms. M. Nakata for her excellent help in preparing the manuscript. MK, YH, SK and TY are funded by the Ministry of Education, Culture, Sports, Science and Technology of Japan. BB was funded by DFG BR2304/5-1, 2304/7-1 and SFB829-B6. There is no competing financial interest. Correspondence should be addressed to MK (mitsumor@vmas.kitasato-u.ac.jp). Communication regarding the Anxa5-KO mouse should be directed to EP (E.Poschl@uea.ac.uk).
Author information
Authors and Affiliations
Contributions
BB and EP established the Anxa5-KO mouse. HU, YN, TL, RT and DR maintained the mouse colony and retrieved the basic reproductive data for the Anxa5-KO mice. YH and HU measured the plasma progesterone levels. HU, TM and TL performed the histological studies, the blood coagulation test and examination of the effects of heparin. SK, TY and RT prepared the histological samples. MK conducted all experiments and prepared the manuscript with HU.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary data
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareALike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Ueki, H., Mizushina, T., Laoharatchatathanin, T. et al. Loss of Maternal Annexin A5 Increases the Likelihood of Placental Platelet Thrombosis and Foetal Loss. Sci Rep 2, 827 (2012). https://doi.org/10.1038/srep00827
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep00827
This article is cited by
-
Recombinant protein diannexin prevents preeclampsia-like symptoms in a pregnant mouse model via reducing the release of microparticles
Frontiers of Medicine (2022)
-
Shifts in the Holstein dairy cow milk fat globule membrane proteome that occur during the first week of lactation are affected by parity
Journal of Animal Science and Biotechnology (2020)
-
Reimagining the antiphospholipid syndrome, an enigmatic thrombophilic disorder, through the looking glass of microscopic imaging
Histochemistry and Cell Biology (2018)
-
Maternal carriers of the ANXA5 M2 haplotype are exposed to a greater risk for placenta-mediated pregnancy complications
Journal of Assisted Reproduction and Genetics (2018)
-
Loss of maternal ANNEXIN A10 via a 34-kb deleted-type copy number variation is associated with embryonic mortality in Japanese Black cattle
BMC Genomics (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.