Abstract
Dendritic cells (DCs) play a crucial role in maintaining the immune system. Though DC-based cancer immunotherapy has been suggested as a potential treatment for various kinds of malignancies, its clinical efficacies are still insufficient in many human trials. Issues that limit the clinical efficacy of DC-based immunotherapy, as well as the difficulty of the industrial production of DCs, are largely due to the limited number of autologous DCs available from each patient. We here established a possible breakthrough, a simple cytokine-based culture method to expand the log-scale order of functional human DCs. Floating cultivation of cord-blood CD34+ cells under an optimized cytokine cocktail led these progenitor cells to stable log-scale proliferation and to DC differentiation. The expanded DCs had typical features of conventional myeloid DCs in vitro. Therefore, the concept of DC expansion should contribute significantly to the progress of DC immunotherapy.
Similar content being viewed by others
Introduction
The World Health Organization's Global Burden of Disease statistics identified cancer as the second-largest global cause of death after cardiovascular disease and predicted that the number of cancer patients would increase1. Although advances in the prevention, diagnostics and treatment of cancer have improved prognoses for cancer patients, cancer is still refractory. We thus need a strong new therapy for advanced malignancies.
Dendritic cells (DCs) have received much attention as a new therapeutic tool for cancer immunotherapy against advanced malignancies. DCs are unique antigen-presenting cells that can efficiently stimulate innate as well as acquired immune responses against pathogens and endogenous cancers. In the past decade, DC-based immunotherapy has been expected to serve as a new therapeutic modality for cancers and infectious diseases. However, relatively limited efficacies have been reported in their clinical studies2. Complex issues, a consequence of too many variables in current clinical studies, must be solved in order to standardize DC-immunotherapy, including DC subtypes, antigen targeting in vivo, doses of DCs and so on3. Among these parameters, the possible critical issue underlying DC-immunotherapy is that limited numbers of DCs (roughly 106 to 108) are available from each patient in clinical studies, even via frequent aphereses for DC progenitor collection4. In our experimental studies on the use of dermal tumor and lung metastasis models, DC-based immunotherapy showed a significant dose response; the optimal dose in both models was at least 106 DCs/30 g, roughly equivalent to 109 DCs per patient according to the weight ratio5,6. Moreover, Provenge, the first FDA-approved autologous cellular therapy, showed a therapeutic outcome in which the median time to disease progression was 31.7 weeks for patients who received more than 1x108 cells per infusion, compared with 12.1 weeks for patients who received fewer cells (p = 0.013)7. Therefore, increasing the number of DCs is expected to improve the efficacy of DC-based immunotherapy in clinical settings. In addition, there is a strong desire to develop technology to increase the number of DCs in order to make the process of DC manipulation less invasive and improve quality control in industrial production.
We have established and optimized a culture method based on the cytokine cocktail FS36 (Flt3-L, SCF, IL-3, IL-6) for DC expansion in mice8. We proved that the expanded DCs had properties that were necessary to obtain therapeutic gains--such as the expression of molecules needed for antigen presentation, endocytotic activity, the potential for inflammatory cytokine/chemokine production and stimulation activity for allogeneic T-cell proliferation--and also showed significant prevention of hypodermic tumors and experimental lung metastasis in vivo. To the best of our knowledge, this is the first demonstration that murine functional DCs can be efficiently expanded ex vivo by more than 3 logs. This technology raised an important question: is the cytokine cocktail used in the study applicable to the expansion of human DCs? As far as we know, only one study addressing this issue has been published. This study used a sequential culture of CD34+ cells in medium containing FS36 followed by an alternative cocktail (FLT3-L, SCF and thrombopoietin) and showed a dramatic expansion (453-fold) in the cell number of progenitors; however, only ∼1x106 DCs were produced from 1x105 CD34+ cells9. Therefore, we here focused on the development of a new technique for the expansion of functional human DCs from cord-blood (CB) CD34+ cells.
There are some reports about CD34+ cell-derived DC-based immunotherapy10,11,12,13,14. It was reported that TNF-alpha is critical for the differentiation and maturation of DCs from CD34+ cells, as it upregulates the GM-CSF receptor while downregulating receptors for other lineage-restricted cytokines15,16. GM-CSF drives the differentiation of monocytes, highly expressing GM-CSF receptor alpha (CD116) into immature DCs and monocytes expressing very low levels of CD116 were shown to be completely refractory to differentiate into DCs and gave rise only to macrophages17. On the other hand, the cytokine cocktail GM/IL-4, a general cytokine cocktail for DC generation without TNF-alpha, could not generate or expand DCs from CD34+ cells. However, the combined use of TNF-alpha and GM-CSF induced the rapid differentiation and activation of DCs from CD34+ cells, so the cell expansion rate would decrease (data not shown). We then proposed the combined use of GM-CSF and stem cell factor (SCF, also known as c-kit ligand). SCF is an early-acting cytokine that binds to receptors that have tyrosine kinase activity; it is expressed on primitive progenitor cells18. Suspension cultures and clonogenic assays of human CD34+ cells both suggest that the addition of SCF leads to higher DC production than with GM-CSF and TNF-alpha19,20,21. Recently, Balan et al. reported a simple two-step culture method for DC expansion from CB CD34+ cells (650-fold). However, the method contained a cell-selection step and used other various cytokines22. For clinical use, a simple method is desired, so we decided to use simple cytokine cocktail GM-CSF and SCF (GM/SCF) for the expansion of DCs and to suppress the rapid activation of progenitor cells by not using TNF-alpha.
We established an optimized culture system, which provided an important advance for generating myeloid DC products from CD34+ cells with high cell numbers, high purity and functionality for use in DC-based immunotherapy for various malignancies.
Results
Kinetics of expansion of CD11c+ cells from CB CD34+ cells
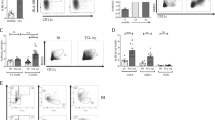
DC expansion regimen is shown in figure 1a. CB CD34+ cells (purity = >95%) were purchased from Lonza (Switzerland) and cultivated for several weeks under the optimized cytokine cocktails. CB CD34+ cells had increased approximately 100,000-fold after 5 weeks of culture under GM/SCF (Figure 1b). When the cultivation was started by GM/IL-4, the yielded cells showed low viability and a low CD11c-positive ratio (data not shown). The CD11c-positive ratio of proliferating cells during cultivation under GM/SCF gradually increased, exceeding 80% after 5 weeks' cultivation (Figure 1c). The expansion rate tended to slow as the CD11c-positive ratio rose. Finally, more than 1010 CD11c+/CD14+ cells were obtained from 105 CB CD34+ cells ( = 105-fold expansion) after 5 weeks' cultivation under GM/SCF (Ex-GSDC) (Figure 1d). More than 108 CD11c+/CD14+ cells were obtained from 105 mobilized PB CD34+ cells ( = 103-fold expansion) after 5 weeks' cultivation under GM/SCF (Supplementary Figure). Moreover, about 5x109 CD11c+/CD14- cells were obtained from 105 CB CD34+ cells ( = 104-fold expansion) by one-week cultivation under GM/IL-4 (GM-CSF and IL-4) after expansion for 4 weeks under GM/SCF (Ex-G4DC) (Figure 1d, e). Flow cytometric analyses revealed the expression of surface markers of each DC (Figure 1f). All DCs in this study were positive for myeloid-associate markers (CD11c and CD11b) and negative for lymphoid-associate markers (B220 and CD3). Ex-GSDC expressed E-cadherin (mediated cell–cell adhesion), however, the expression on Ex-G4 DC and Md-DC were low or negative. These experiments were repeated at least three times in 3 individuals.
Cytokine-based expansion of CB CD34 + cell-derived CD11c + cells.
(a) The regimen of cell expansion from CB CD34+ cells. (b) Growth curve of total cells from CB CD34+ cells. After 4 weeks of cultivation under GM/SCF (black line), culture medium was subsequently replaced with GM/IL-4 (red line) for 1 week. GM/IL-4 could not expand the cells. (c) CD11c-positive ratios of expanded cells under GM-CSF and SCF. The ratio of proliferating cells during cultivation under GM/SCF gradually increased, exceeding 80% after 5 weeks' cultivation. The cells cultured under GM/IL-4 for a week after 4 weeks' cultivation under GM/SCF also showed a high CD11c-positive ratio. (d) Growth curve of CD11c+ cells from CB CD34+ cells. These experiments were repeated seven times in 5 individuals (GM/SCF) and three times in 3 individuals (GM/IL-4). (e) The numbers of CD11c+/CD14+ and CD11c+/CD14- DC generated from each culture. (f) FACS profiles of the DC products. These experiments were repeated at least three times in 3 individuals.
Characterization of expanded CD11c+ cells
Next, we assessed whether or not CD11c+ cells yielded by the expansion method from CB CD34+ cells (Ex-GSDC and Ex-G4DC) might meet typical features of DCs via a conventional culture method (CD14+ monocyte-derived DC: md-DC).
1) Morphology
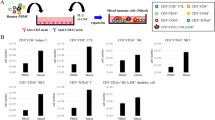
As are seen in md-DC, typical dendrites were found in both Ex-GSDC and Ex-G4DC under microscope 2 days after cultivation under medium containing stimulators: lipopolysaccharide (LPS: 1 µg/ml), OK-432 (0.5 KE/ml), or rSeV/dF (MOI = 50). rSeV/dF-activated DCs are shown in Figure 2a.
In vitro characterization of expanded human DCs.
(a) Morphology of rSeV-activated DCs. Wright-Giemsa–stained cytocentrifuge preparations are shown. Bars in panels = 30 um. (b) FACS analyses assessing the expression of typical surface markers. DCs with or without further stimulus were subjected to FACS analyses. A bar graph indicating the corresponding mean fluorescent intensity (MFI) containing data from four independent experiments is shown.
2) Surface markers
Flow cytometric analyses revealed the expression of typical surface markers of each DC (Figure 2b and Supplementary Table). Although the expression level of each antigen varied among the DCs, all types of cells expressed surface markers needed for antigen presentation and chemokine receptors such as MHC class II (HLA-DR), CD86, ICAM-1, CCR5 and CCR7. Expression of MHC Class II was clearly upregulated by rSeV infection. Ex-GSDC and Ex-G4DC showed higher levels of ICAM-1 expression than md-DC. All data were analyzed after CD11c-positive gating.
3) Production of inflammatory cytokines and chemokines
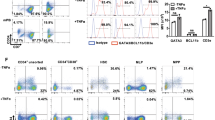
All types of DCs commonly expressed typical inflammatory cytokines and chemokines in response to various stimulators, including the F-gene-deleted non-transmissible recombinant Sendai virus (rSeV/dF) recognized by RIG-I23, LPS by Toll-like receptor 4 (TLR-4), or Streptococcal preparation OK-432 (a GMP grade agent for the functional maturation of DCs) by TLR-4 and β2 integrins24,25 (Figure 3a). Although Ex-GSDC failed to produce TNF-alpha or IL-12p70 (needed for Th1 induction), Ex-G4DC expressed these cytokines as seen in md-DC stimulated by OK-432. Both Ex-DCs activated by LPS produced lower levels of IL-10 (a cytokine for Th2 induction) than md-DC activated by LPS did. rSeV/dF could not induce a high production level of each cytokine and chemokine from all types of DCs and these findings were similar to those in our previous study6,8. rSeV/dF induced spontaneous maturation and activation of DCs, however, their phenotype is not equal to those seen in the treatment with LPS26.
Assessment of functions that are typically seen in DCs.
(a) Expression of typical human inflammatory cytokines/chemokines of monocyte-derived DCs and expanded DCs in response to various stimuli. These six panels were assessed by the Cytometric Bead Array (CBA) system and contain data from three independent experiments. (b) FITC-dextran uptake assay assessing endo-/phagocytotic activity, a typical feature of antigen-presenting cells like DCs. DCs were stimulated by each stimulus and then exposed to 1 mg/ml of FITC–dextran for 30 min at 4 or 37 degrees Celsius. The uptake was expressed MFI between cell samples incubated at 37 and 4 degrees Celsius. This experiment was performed three times. (c) A graph showing MLR activity for allo-antigen by each immature DC or activated DC by SeV/dF, LPS, or OK-432. This experiment was performed three times.
4) Endo-/phagocytotic activity
Next, we assessed the endo-/phagocytotic activity, a typical feature of antigen-presenting cells like DCs, by an FITC-dextran (M.W. = 40,000) uptake assay (Figure 3b). Repeated experiments demonstrated that each unstimulated immature DC showed similar uptake activity that was impaired by rSeV/dF infection or LPS, findings similar to those of our previous study6. Although a significant decrease in phagocytotic activity was not seen in Ex-GSDC, a tendency toward a decrease was seen. Each OK-432-stimulated DC showed higher phagocytotic activity than those stimulated by rSeV/dF or LPS.
5) T-lymphocyte stimulation activity
The assay for mixed leukocyte reaction (MLR) against allogeneic antigen (CD3+ T-cells from a volunteer) demonstrated the alloantigen-specific T-lymphocyte proliferation by each DC stimulated with or without rSeV/dF, LPS, or OK-432. This, in turn, suggested the stimulating activity of all types of DCs to alloantigen-specific T-cell proliferation (Figure 3c). Especially, Ex-G4DC and md-DC stimulated by OK-432 showed strong activity.
Discussion
The ultimate needs for sufficient numbers of functional DCs in clinical settings, in view of both therapeutic efficacy and industrial production, have forced us to develop an efficient and representative method for mass producing functional DCs. We established and optimized a culture method for DC expansion in mice using a two-step culture8. However, the culture method couldn't be extended to human DC expansion. We then established and optimized a new culture method for human DC expansion: a 5-week expansion and differentiation under GM-CSF and SCF (GM/SCF); and a two-step culture method: a 4-week expansion and differentiation under GM/SCF followed by one week of cultivation under GM/IL-4. The expansion rates varied according to the cell sources. The cells showing greater expansion were CB CD34+ cells; they increased approximately 100,000-fold after 5 weeks of culture and >80% of expanded cells expressed CD11c. To the best of our knowledge, this is the first demonstration that human functional DCs derived from CD34+ cells can be efficiently expanded ex vivo by more than 5-logs. In some clinical reports, the administration of 1x107 DCs was effective27,28, so the administration of 1x108 DCs (10-fold of the present clinical dose in number) may be sufficient to improve therapeutic outcomes. Even so, a DC-expansion method is important for the preparation of vaccinations in large quantities.
As are seen in conventional DCs, these expanded DCs showed dendrites after maturation and endocytotic activities. Expanded DCs also expressed HLA-DR, adhesion molecules and co-stimulatory molecules and produced inflammatory cytokines/chemokines as well as conventional DCs did. However, the DCs failed to up-regulate some antigens following addition of each stimulator. Obermajer N. et al. reported that maturation coincides with the de-adhesion of DCs and formation of cell clusters29. Our all culture systems are “floating cultivation” using MPC-treatment plates. Therefore, we consider that each DC in this study may different from conventional “adherent” DCs and be activated. In addition, the cytokines produced by each DC generated from different culture conditions are quite different. We have reported that the responses of some cytokines including IL-12/p70 and TNF-alpha to rSeV/dF recognized by RIG-I, LPS by TLR4, poly I:C by TLR3, CpG-DNA by TLR9 and R848 by TLR7 were different among DCs generated from different culture system (non-adherent, expansion or conventional methods)8. These data suggested that the cytokines produced by DC might be in accordance with the expression patterns of the receptors (i.e. TLRs) on DCs.
Additionally, there are some reports that OK-432 but LPS strongly stimulates DCs to secrete Th1 cytokine (IL-12p70) without Th2 cytokine (IL-10) production30,31. These data suggested that DCs stimulated with OK-432 might have favorable characteristics to induce CTLs specific to antigen peptide. Because of high induction of Th1 cytokines and low induction of Th2 cytokines, our data also suggested that OK-432 might show the best clinical efficacy among activators tested.
Ex-GSDC and Ex-G4DC showed higher ICAM-1 expression than did md-DC. ICAM-1 (Inter-Cellular Adhesion Molecule 1), also known as CD54, binds to macrophage adhesion ligand-1 (Mac-1), leukocyte function-associated antigen-1 (LFA-1) and fibrinogen. These three proteins are generally expressed on endothelial cells and leukocytes and they bind to ICAM-1 to facilitate transmigration across vascular endothelia in processes such as extravasation and the inflammatory response. The classical definition of the immunological synapses is based on a cell–cell interface in which LFA-1–ICAM-1 interactions and talin form a ring around a central cluster of TCR–MHCp (T-cell receptor–major histocompatibility complex molecule–peptide complex) interactions and protein kinase C-θ32,33,34,35. Moreover, according to a report by Eric J. Small et al., they correlated the expression of costimulatory molecules with potency in the allo-MLR in 81 consecutive lots of Provenge and found that potency correlated most strongly with CD54 expression. In addition, cell-sorting experiments revealed that all antigen-presenting activity resided in the CD54+ population. Based on these observations, they selected CD54 expression as a marker of dendritic cells and product potency7. High ICAM-1 expression levels would support the stabilized and more efficient interaction between DC and T lymphocyte in vivo.
Functionally, the mixed lymphocyte reaction (MLR) assay revealed that expanded DCs could stimulate allogeneic T-cell proliferation to the same extent as conventional DCs. Taking these findings together, we concluded that cells expanded and differentiated from CB CD34+ cells could be categorized into functional DCs nearly equivalent to those obtained from the conventional method. In addition, we succeeded in expanding the CD11c+ cells from enriched monocyte by cultivation under GM/SCF (data not shown).
Several studies have assessed the expansive ability of cytokines for DCs from CD34+ cells. However, as far as we know, the most recent study attempting to expand human DCs was published in 2009 by Balan et al., who used a relatively complex cocktail of cytokines (GM-CSF, TNF-alpha, IL-4, TPO, SCF and FLT3-L) and two-step cultivation containing a monocyte selection step22. Clinical use necessitates a simple culture system without complex steps such as a magnetic-bead method for selecting cells, flow cytometry-cell sorting, or adherent selection. Although others reported some methods for DC induction from CD34+ cells without cell selection, the purity of DC was relatively low (<50–60%)7,11,12,36,37,38, whereas our method broke through this problem (>80%). For mass production and quality control, a “DC bank” based on the cord-blood bank could be established.
We demonstrated that expanded DCs showed characteristics that, from the standpoints of morphology, surface markers and biological functions in vitro, were similar to those seen in conventional DCs. If CTLs were the main effector of DC-based immunotherapy against cancer, Ex-G4DC activated by OK-432 might show the best clinical efficacy among these expanded DCs because the cells produced IL-12p70 and showed strong T-lymphocyte stimulation activity. However, we reported that the main effector of antitumor immunity is CD4+ T cells and NK cells39. Upon administration, the vaccine is thought to induce an antigen-specific T-cell response and NK activation against the tumor. The therapeutic gains of both expanded DCs, Ex-GSDC and Ex-G4DC, have to be assessed.
A problem that needs to be solved for clinical use is that the expanded DCs yielded from CB CD34+ cells were generally allogeneic to patients. Though some reported that allogeneic DCs were effective, autologous DCs were needed to achieve efficient clinical efficacies40,41 and safety. Although we could also expand DCs from PB CD34+ cells (3-log), the number of DCs might not be enough to treat various malignancies because of very low numbers of CD34+ cells and the difficulty of collecting a sufficient number of progenitor cells. To obtain a sufficient number of DCs, we attempted to extend this expansion method using CD34+ cells to expansion from peripheral blood mononuclear cells (PBMCs). We then succeeded in expanding DCs from PBMC by an easy new method that achieved an expansion rate = ∼ 1x107 DCs from 1 ml PB (patent pending).
In summary, we here report optimized one-step and two-step culture methods to generate highly expanded functional myeloid DCs from CB CD34+ cells. The concept and technology of log-scale expansion of functional DCs would significantly contribute not only to improved therapeutic efficacies but also to industrial mass production of functional autologous DCs for more efficient DC-based immunotherapy. DC expansion technology will improve cancer therapies and alleviate patients' burden of apheresis.
We should optimize other parameters of the DC vaccine and enhance the development of immunotherapy. In addition, future clinical studies should utilize standard criteria for clinical response and require validation in increased numbers of patients.
Methods
Cells and rSeV/dF
Human cord-blood (CB) CD34+ cells were purchased from Lonza (Switzerland). The preparation, recovery, titration and storage of F-defective and non-transmissible recombinant SeV used in this study (SeV/dF) were performed as previously described42. Virus yield is expressed in cell infectious units (CIU)42,43.
Generation of expanded DCs
Conventional DCs were obtained from peripheral blood CD14+ cells as described previously, with minor modification. Briefly, CD14+ cells were cultured under GM/IL-4 (100 ng/ml GM-CSF and 50 ng/ml IL-4) in IMDM medium containing 10% FBS and 1% penicillin and streptomycin. CD14+ monocytes were obtained by negative selection. Briefly, peripheral blood mononuclear cells (PBMC) were obtained using a Ficoll gradient separation purchased from the Australian Red Cross Blood Service (Melbourne, Vic., Australia). Cells were washed in PBS and lineage antigen-positive (CD2, CD3, CD19, CD20, CD56, CD66b, CD123, Glycophorin A) cells were removed by using the EasySep human monocyte enrichment kit without CD16 depletion (StemCell Technologies, Canada). These lineage-negative CD14+ cells were cultured under 100 ng/ml human GM-CSF (Peprotech, USA) and 50 ng/ml human IL-4 (Peprotech) in IMDM medium. Each cytokine was dissolved in PBS containing 0.1% BSA in 100 times the density used. For expansion, CD34+ cells were cultured under GM/SCF (100 ng/ml GM-CSF and 50 ng/ml SCF) or GM/IL-4 (100 ng/ml GM-CSF and 50 ng/ml IL-4) in IMDM medium. The medium was exchanged every 3 or 4 days. Cells were cultured in the MPC treatment plate (MD6 with Lid Low-Cell Binding; Nalge Nunc International, Japan).
Wright-Giemsa staining
The DCs were morphologically assessed using the Wright-Giemsa staining method. Cells were prepared on slides by Cytospin (Shandon Southern, UK) and stained with Wright-Giemsa stain. The cell morphology was examined under a light microscope.
Flow cytometric analysis
After 5 week's cultivation or 2 days after stimulation, cells (1×105) were stained with the following FITC-, PE-, or PE-Cy5-conjugated monoclonal antibodies (mAbs): CD1a, CD1d, CD3, CD11b, CD11c, CD14, CD33, CD40, CD45R (B220), CD54 (ICAM-1), CD80, CD83, CD86, CD123 (IL-3Ra), CD195 (CCR5), CD197 (CCR7), CD207 (Langerin), CD324 (E-cadherin), HLA-ABC, HLA-DR (Pharmingen, USA). The appropriate conjugated isotype-matched IgGs were used as controls. Cells were analyzed using FACScalibur with the use of CellQuest software (Becton Dickinson, USA).
Fluorescein isothiocyanate (FITC)-dextran uptake
Cells were suspended in IMDM with 10% FBS and incubated with 1 mg/ml of FITC-dextran (M.W. = 40,000, Sigma-Aldrich, Tokyo, Japan) for 30 min under separate conditions, at 4oC and 37oC. The cells were washed three times with ice-cold phosphate-buffered saline (PBS) and labeled on ice with PE-conjugated mAb for CD11c. CD11c-positive mean fluorescent intensity (MFI) of FITC was analyzed by FACScalibur. The uptake was measured at 2 days after stimulation and was calculated as the change in MFI between cell samples incubated at 37oC and those incubated at 4oC.
Allogeneic mixed leukocyte reactions (allo-MLRs)
Allogeneic CD3+ T-cells were obtained from a volunteer. After Ficoll separation (GE Healthcare Bio-Sciences AB, Sweden), cell surface antigen-positive (CD14, CD16, CD19, CD56, glycophorin A) cells were removed by using the EasySep Human T-Cell Enrichment kit (StemCell Technologies, Canada); these served as responder cells. In-vitro-generated immature expanded DCs, as well as rSeV/dF-DCs, LPS-DCs and OK-432-DCs stimulated on day 35, were collected on day 37. These DCs were treated with 20 ug/ml MitomycinC (MMC) for 1 hour at 37oC and then used as stimulator cells. Allogeneic responder cells (1×105 cells/wells) were cultured in duplicate or triplicate in a 96-well round-bottom microplate with different numbers of stimulator APCs (APC-to-T cell ratios were 1∶10, 1∶100 and 1∶1000). Cultures were maintained in a humidified atmosphere at 37oC and 5% CO2. The thymidine analogue BrdU was added on day 4 followed by quantitation of incorporated BrdU after a further 2 hours of culture using an ELISA-based cell proliferation kit (BrdU colorimetric, 1647229, Roche,Germany) according to the manufacturer's protocol.
Cytokine assay
The conventional/expanded DCs were cultured with rSeV/dF (MOI = 50), LPS (1 ug/ml), or OK-432 (0.5 KE/ml) for 2 days. The culture media were subjected to concentrations of human IL-1 beta, IL-6, IL-8, IL-10, IL-12p70 and TNF-alpha by the Cytometric Bead Array (CBA) Human Inflammation Kit (BD Biosciences, USA) using FACScalibur with the use of CellQuest software (Becton Dickinson, USA).
Statistical analysis
All data were expressed as the mean ± SEM and were evaluated statistically by one-way ANOVA. The statistical significance of differences was determined using the Dunnett's test and P<0.05 was considered statistically significant.
References
Mathers, C. D. & Loncar, D. Projections of Global Mortality and Burden of Disease from 2002 to 2030. PLoS Med. 11, e442 (2006).
Rosenberg, S. A., Yang, J. C. & Restifo, N. P. Cancer immunotherapy: moving beyond current vaccines. Nat. Med. 10, 909–915 (2004).
Figdor, C. G., de Vries, I. J., Lesterhuis, W. J. & Melief, C. J. Dendritic cell immunotherapy: mapping the way. Nat. Med. 10, 475–480 (2004).
Harada, Y. & Yonemitsu, Y. Recent developments in patented DC-based immunotherapy for various malignancies. Recent Patents on Regenerative Medicine 1, 72–87 (2011).
Tatsuta, K., et al. Complete elimination of established neuroblastoma by synergistic action of gamma-irradiation and DCs treated with rSeV expressing interferon-beta gene. Gene Ther. 16, 240–251 (2009).
Kato, T., et al. RIG-I helicase-independent pathway in sendai virus-activated dendritic cells is critical for preventing lung metastasis of AT6.3 prostate cancer. Neoplasia. 12, 906–914 (2010).
Small, E. J., et al. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J. Clin Oncol 18, 3894–3903 (2000).
Harada, Y., et al. Cytokine-based log-scale expansion of functional murine dendritic cells. PLos ONE. 18 4(8), e6674 (2009).
Bontkes, H. J., De Gruijl, T. D., Schuurhuis, G. J., Scheper, R. J., Meijer, C. J. & Hooijberg, E. Expansion of dendritic cell precursors from human CD34+ progenitor cells isolated from healthy donor blood; growth factor combination determines proliferation rate and functional outcome. J. Leukoc Biol. 72, 321–329 (2002).
Fay, J. W., et al. Long-term outcomes in patients with metastatic melanoma vaccinated with melanoma peptide-pulsed CD34+ progenitor-derived dendritic cells. Cancer Immunol. Immunother. 55, 1209–1218 (2006).
Banchereau, J., et al. Immune and clinical responses in patients with metastatic melanoma to CD34 progenitor-derived dendritic cell vaccine. Cancer Res. 61, 6451–6458 (2001).
Di Nicola, M., et al. Boosting T cell-mediated immunity to tyrosinase by vaccinia virus-transduced, CD34-derived dendritic cell vaccination: A phase I trial in metastatic melanoma. Clin Cancer Res 10, 5381–5390 (2004).
Di Nicola, M., et al. Clinical protocol. Immunization of patients with malignant melanoma with autologous CD34 cell-derived dendritic cells transduced ex vivo with a recombinant replication-deficient vaccinia vector encoding the human tyrosinase gene: a phase I trial. Hum Gene Ther. 14, 1347–1360 (2003).
Mackensen, A., et al. Phase I study in melanoma patients of a vaccine with peptide-pulsed dendritic cells generated in vitro from CD34+ hematopoietic progenitor cells. Int J. Cancer. 86, 385–92 (2000).
Warren, M. K., Rose, W. L., Cone, J. L., Rice, W. G. & Turpin, J. A. Differential infection of CD34+ cell-derived dendritic cells and monocytes with lymphocyte-tropic and monocyte-tropic HIV-1 strains. J. Immunol. 158, 5035–5042 (1997).
Caux, C., Saeland, S., Favre, C., Duvert, V., Mannoni, P. & Banchereau, J. Tumor necrosis factor-alpha strongly potentiates interleukin-3 and granulocyte-macrophage colony-stimulating factor-induced proliferation of human CD34+ hematopoietic progenitor cells. Blood. 75, 2292–2298 (1990).
Conti, L. & Gessani, S. GM-CSF in the generation of dendritic cells from human blood monocyte precursors: recent advances. Immunobiology. 213, 859–870 (2008).
Rondelli, D., et al. Rapid induction of CD40 on a subset of granulocyte colonystimulating factor-mobilized CD34+ blood cells identifies myeloid committed progenitors and permits selection of nonimmunogenic CD40+ progenitor cells. Blood 94, 2293–2300 (1999).
Lyman, S. D. & Jacobsen, S. E. C-Kit ligand and FLT3 ligand: stem/progenitor cell factors with overlapping yet distinct activities. Blood. 91, 1101–1134 (1998).
Szabolcs, P., Moore, M. A. & Young, J. W. Expansion of immunostimulatory dendritic cells among the myeloid progeny of human CD34+ bone marrow precursors cultured with c-Kit ligand, granulocyte-macrophage colonystimulating factor and TNF-alpha. J. Immunol. 154, 5851–5861 (1995).
Ratta, M., et al. Generation and functional characterization of human dendritic cells derived from CD34+ cells mobilized into peripheral blood: comparison with bone marrow CD34+ cells. Br. J. Haematol. 101, 756–765 (1998).
Balan, S., Kale, V. P. & Limaye, L. S. A simple two-step culture system for the large-scale generation of mature and functional dendritic cells from umbilical cord blood CD34+ cells. Transfusion. 49, 2109–2121 (2009).
Kato, T., Ueda, Y., Kinoh, H., Tsukada, K., Ichikawa, T. & Yonemitsu, Y. Pathogen-related signal transduction pathways of dendritic cells: perspectives for cancer immunotherapy. Front. Biosci. 3, 133–137 (2008).
Okamoto, M., et al. Expression of toll-like receptor 4 on dendritic cells is significant for anticancer effect of dendritic cell-based immunotherapy in combination with an active component of OK-432, a streptococcal preparation. Cancer Res. 64, 5461–5470 (2004).
Nakahara, S., Tsunoda, T., Baba, T., Asabe, S. & Tahara, H. Dendritic cells stimulated with a bacterial product, OK-432, efficiently induce cytotoxic T lymphocytes specific to tumor rejection peptide. Cancer Res. 63, 4112–4118 (2003).
Shibata, S., et al. Induction of efficient antitumor immunity using dendritic cells activated by recombinant Sendai virus and its modulation by exogenous IFN-beta gene. J Immunol. 177, 3564–3576 (2006).
Nagayama, H., et al. Results of a phase I clinical study using autologous tumour lysate-pulsed monocyte-derived mature dendritic cell vaccinations for stage IV malignant melanoma patients combined with low dose interleukin-2. Melanoma Res. 13, 521–530 (2003).
Trefzer, U., et al. Vaccination with hybrids of tumor and dendritic cells induces tumor-specific T-cell and clinical responses in melanoma stage III and IV patients. Int J Cancer. 110, 730–740 (2004).
Obermajer, N., Svajger, U., Bogyo, M., Jeras, M. & Kos, J. Maturation of dendritic cells depends on proteolytic cleavage by cathepsin X. J Leukoc Biol. 84, 1306–1315 (2008).
Kuroki, H., et al. Streptococcal preparation OK-432: a new maturation factor of monocyte-derived dendritic cells for clinical use. Cancer Immunol Immunother. 52, 561–568 (2003).
Nakahara, S., Tsunoda, T., Baba, T., Asabe, S. & Tahara, H. Dendritic cells stimulated with a bacterial product, OK-432, efficiently induce cytotoxic T lymphocytes specific to tumor rejection peptide. Cancer Res. 63, 4112–4118 (2003).
Monks, C. R., Freiberg, B. A., Kupfer, H., Sciaky, N. & Kupfer, A. Threedimensional segregation of supramolecular activation clusters in T cells. Nature. 395, 82–86 (1998).
Dustin, M. L., et al. A novel adapter protein orchestrates receptor patterning and cytoskeletal polarity in T cell contacts. Cell 94, 667–677 (1998).
Dustin, M. L. & Shaw, A. S. Costimulation: building an immunological synapse. Science 283, 649–650 (1999).
Dustin, M. L., Tseng, S. Y., Varma, R. & Campi, G. T cell–dendritic cell immunological synapses. Curr Opin Immunol. 18, 512–516 (2006).
Soligo, D., Lambertenghi Deliliers, G., Quirici, N., Servida, F., Caneva, L. & Lamorte, G. Expansion of dendritic cell derived from human CD34+ cells in static and continuous perfusion cultures. Br J. Haematol. 101, 352–363 (1998).
Curti, A., Fogli, M., Ratta, M., Tura, S. & Lemoli, R. M. Stem cell factor and FLT3-ligand are strictly required to sustain the long-term expansion of primitive CD34+DR- dendritic cell precursors. J. Immunol. 166, 848–854 (2001).
Sioud, M. & Fløisand, Y. TLR agonists induce the differentiation of human bone marrow CD34+ progenitors into CD11c+ CD80/86+ DC capable of inducing a Th1-type response. Eur J. Immunol. 37, 2834–2846 (2007).
Komaru, A., et al. Sustained and NK/CD4+ T cell-dependent efficient prevention of lung metastasis induced by dendritic cells harboring recombinant Sendai virus. J. Immunol 183, 4211–4219 (2009).
Yasuda, T., et al. Superior anti-tumor protection and therapeutic efficacy of vaccination with allogeneic and semiallogeneic dendritic cell/tumor cell fusion hybrids for murine colon adenocarcinoma. Cancer Immunol Immunother, 56, 1025–1036 (2007).
Merrick, A., Diaz, R. M., O'Donnell, D., Selby, P., Vile, R. & Melcher, A. Autologous versus allogeneic peptide-pulsed dendritic cells for anti-tumour vaccination: expression of allogeneic MHC supports activation of antigen specific T cells, but impairs early naive cytotoxic priming and anti-tumour therapy. Cancer Immunol Immunother, 57, 897–906 (2008).
Li, H. O., et al. A cytoplasmic RNA vector derived from nontransmissible Sendai virus with efficient gene transfer and expression. J. Virol. 74, 6564–6569 (2000).
Yonemitsu, Y., et al. Efficient gene transfer to the airway epithelium using recombinant Sendai virus. Nat Biotechnol. 18, 970–973 (2000).
Acknowledgements
The authors would like to thank Drs. Mariko Yoshizaki, Akihiro Tagawa, Takumi Kanaya, Hiroshi Ban and Takashi Hironaka for their excellent technical assistance with the construction and large-scale production of rSeV vectors. This work was supported in part by a Grant-in-Aid (to YY) from the Japanese Ministry of Education, Culture, Sports, Science and Technology; and by Research Grants from the Sankyo Foundation of Life Science (to YY) and from the Uehara Memorial Foundation (to YY).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: YH YU ST TI YY. Performed the experiments: YH YON YU TFO. Analyzed the data: YH YU YY. Contributed reagents/materials/analysis tools: YH YU AI MH. Wrote the paper: YH.
Ethics declarations
Competing interests
Dr. Yonemitsu was a member of the Scientific Advisory Boards of DNAVEC Corporation and Tella, Inc. There are no conflicts of interest for all other authors.
Electronic supplementary material
Supplementary Information
Supplemantal figure and table
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareALike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Harada, Y., Okada-Nakanishi, Y., Ueda, Y. et al. Cytokine-based high log-scale expansion of functional human dendritic cells from cord-blood CD34-positive cells. Sci Rep 1, 174 (2011). https://doi.org/10.1038/srep00174
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep00174
This article is cited by
-
Umbilical cord blood-derived CD11c+ dendritic cells could serve as an alternative allogeneic source of dendritic cells for cancer immunotherapy
Stem Cell Research & Therapy (2015)
-
Interaction of dendritic cells and T lymphocytes for the therapeutic effect of Dangguiliuhuang decoction to autoimmune diabetes
Scientific Reports (2015)
-
Dendritic Cell Immune Therapy to Break or Induce Tolerance
Current Stem Cell Reports (2015)
-
Assessment of genetic markers and glioblastoma stem-like cells in activation of dendritic cells
Human Cell (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.