Key Points
-
Suggests genetic testing for dental developmental disorders is becoming accessible and affordable beyond the research setting.
-
Suggests whole genome sequencing is becoming the method of choice.
-
Highlights that incidental non-dental findings may be discovered and need explanation.
-
Proposes that counselling should be offered for dental genetic testing.
Abstract
Genetic testing for serious illness and disease is becoming increasingly embedded in NHS healthcare. It can confirm a clinical diagnosis or guide therapy. Genetic testing for dental developmental disorders has moved beyond the realms of rarified grant-funded research groups and is now sufficiently rapid and affordable to be offered as part of a clinical service in some dental teaching hospitals. The first presentation of some genetic diseases may be in the dental surgery, so the family dentist should hone their diagnostic skills to identify patients who would benefit from referral to a genetics service. While diagnosis may sometimes guide treatment, there are now examples where it can even lead to cure. This article aims to describe some concepts and issues that a dentist should consider when referring for testing for a genetic dental disorder, and proposes that this subject area should be expanded in the dental undergraduate and postgraduate curricula in the UK.
Similar content being viewed by others
Introduction
The genetic control of your 32 teeth is contained in your 23 chromosomes, and it is becoming faster, cheaper and easier to unlock this information. Direct-to-consumer gene-testing kits are now available in high street pharmacies, where a saliva sample contains enough DNA to allow in-depth genetic testing. Only a few weeks after posting off the sample the consumer receives a personalised report on many aspects of their health and development, from cancer risks to earwax type. Few dentists consider themselves experts in genetics, but members of the public now have access to their own genetic information, and may expect their healthcare providers to help interpret their results.
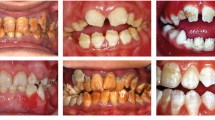
Every day in dental practice patients will present with disorders of development which are genetic in origin, whether it is a mild frosty enamel hypomineralisation defect, absent mandibular premolars, or the troublesome stigmata of a more severe amelogenesis imperfecta (AI). A patient may ask their dentist whether a dental defect is genetic in origin or whether a test is available. Indeed the dentist may suspect that dental problems could be a manifestation of an underlying genetic syndrome. The dentist then needs to consider a number of very sensitive issues which are removed from the daily decisions made in clinical practice (Box 1).
Readers of the BDJ have been kept informed of many recent advances in the knowledge of genetic dental disorders, including AI, hypodontia and supernumerary teeth.1,2,3 These gene discoveries are usually the result of groups of researchers investigating carefully selected families. Echoing the advances in medical genetics, the context of dental genetic investigation is now changing from research to more widely-available diagnostic services.
Saliva DNA tests are available to detect oral HPV infection, which has been established as conferring a significantly increased risk for oral and oro-pharyngeal cancer.4 Periodontal disease susceptibility can be influenced by genetic background, forming the basis of some commercially available tests.5 Even the gene for a 'sweet tooth' has been reported, with possible implications for controlling diabetes, obesity, and caries.6 The widely-held public belief that bad teeth runs in families may actually have some scientific basis.7
Over 1,000 genes are known to be associated with tooth development,8 and the 'human dentome' is still far from being completed. Given the complexity of oral and dental genetics, how is the family dentist to engage with the new discoveries to help their patients? Some aspects of this are introduced in this paper.
Dentists making the early diagnosis
The family dentist may be the first healthcare provider to have the opportunity to diagnose a genetic condition from the dental presentation alone. Good examples are; hypophosphatasia (HPP) – where primary teeth can exfoliate prematurely with intact roots, X-linked hypophosphataemic rickets (XLH) – where spontaneous abscesses can occur in apparently sound primary teeth, and ectodermal dysplasia (ED) – where primary teeth can be missing or conical, sometimes in the absence of obvious facial features of the condition. Syndromes where the mildest form or carrier status is manifested by dental changes alone warrant further investigation.
Why test for genetic dental disorders?
For the very few single-gene ('monogenic') disorders that are purely dental, confirmation of a clinical diagnosis by identification of a molecular change will not change patient management – either dental or medical. This covers most types of AI, and those types of dentinogenesis imperfecta (DI) that are dental-only. However, there is a rare form of AI with characteristic clinical and radiographic appearance that is known to be caused by a gene change that also predisposes to nephrocalcinosis.9 Likewise, some patients presenting only with DI have been shown to carry a change in the causative gene for the brittle bone disease osteogenesis imperfecta.10
A dentist who is alert to the potential syndromic implications of dental findings could transform the medical care for the affected patient, and sometimes their wider family.
A cure for dental genetic disorders?
Three of the genetic conditions mentioned above now have the potential for being cured. The deficient level of the enzyme alkaline phosphatase responsible for the skeletal and dental manifestations of HPP is now treatable with artificial enzyme therapy.11 Correction of the skeletal rickets can be dramatic, but the impact on defective cementum formation and tooth exfoliation is not yet clear.
Similarly, the severe skeletal rickets seen in children with XLH has been shown to respond to a drug which targets the excessive accumulation of the defective gene product. This drug was licenced for use in the US in 2018 but is still under consideration in the UK.12
The classic form of ED, the X-linked hypohydrotic form XLHED, is currently the subject of clinical trials of a genetically corrected form of the altered gene product ectodysplasin.13 While results of human studies have not yet been published, one may hope for improvements in some non-dental features of ED, but for teeth to grow treatment would have to be given in utero.
Paradoxically, while these conditions may all present initially with dental problems, currently it seems to be the non-dental features that are rescued by therapy. This is simply explained by the knowledge that dental hard structures are formed early in life and are not constantly re-modelled and replaced in the same way as skeletal bone.
Whole genome sequencing
Sequencing a person's entire genome to aid in diagnosis of a disease is rapidly replacing the traditional strategies of targeted sequencing of candidate genes. Whole genome sequencing (WGS) decodes the entire nose-to-tail string of nucleotides, including the sequences that are genes and the intervening sequences that used to be thought of as 'junk DNA'. An abbreviated version of WGS is being used by many genetics diagnostic services, just looking at the gene-coding sections of DNA, and is called whole exome sequencing (WES).14
WGS and WES do not only produce findings directly related to the condition being investigated ('pertinent findings', [PFs]) but information which was not initially being searched for ('incidental findings', [IFs]). The implications of these IFs are the subject of lively debate in the world of medical genetics. Should they not be looked at, even though the data is captured during WGS? Should only some important disease-causing genes be checked? Who should decide whether to look – patient, doctor, scientist or biostatistician? Who should decide whether to disclose the results, and to whom? Who should own the data? Can an individual ask for a CD containing their own genome sequence, to do with as they wish?15
Screening for serious conditions
There is a need within the NHS for the creation of clear guidance for patients and clinicians who are involved in genomic screening. 'Sequencing a genome does not equate to screening a genome'.16
A difference of opinion has arisen between medical geneticists in the United States and Europe. The American College of Medical Genetics and Genomics recommends actively seeking certain IFs. Screening a panel of 57 agreed genes is mandatory, regardless of the disease being tested for. This panel includes a number of cancer-causing gene variants, hypercholesterolemia, and cardiac arrhythmia conditions. What most of these conditions have in common is onset in adulthood, while a genetic condition under investigation often presents during childhood. Any mutations identified in this panel must be reported to the responsible clinicians.17 By contrast, the European Society of Human Genetics recommends a more targeted approach to analysis of WGS data, actively not looking at genomic regions other than those likely to be relevant to the disorder under investigation. The UK Association of Genetics Nurses and Counsellors recommends that children should not be opportunistically tested for adult-onset conditions and patients should be allowed to opt out of receiving IFs.18
Most dental teaching hospitals are allied to medical institutions with genetics departments which are now potentially able to offer WGS/WES on a service basis. The realities of funding and clinical importance mean these WGS facilities are prioritised for more serious and life-threatening diseases than dental anomalies. Any centre offering dental genetic testing must consider aspects of probity and information-sharing, particularly with respects to PFs and IFs (Box 2).
As with histopathology results for oral biopsies, pertinent results of dental genetic investigation should be made available to a patient's dentist. This forms part of the clinical record and will be accessible to the wider dental team. Protection of personal genetic information is arguably among the most sensitive areas of medical data protection, and we should all be aware of the need for confidentiality.
There is a need for agreement at the beginning of any dental diagnostic investigation as to how much detail the family dentist should be given, and how this information should be held.
Dental genetics clinic example
As far as the authors are aware, the first dedicated dental genetics service in the UK is run at Guy's and St Thomas' Hospitals in London. Diagnostic clinics are attended by the specialties of clinical genetics, paediatric dentistry, orthodontics and craniofacial biology. The patients seen are chosen because they have unusual dentitions thought to have a genetic cause, but which do not fit clearly into any known malformation syndrome. A diagnostic strategy is suggested by the phenotype (clinical features) and the family pedigree. In its first three years, positive genetic diagnosis has been confirmed by WES in several families, finding unexpected pathological changes in genes including PITX3 associated with severe hypodontia and ocular defects, and ANKRD11 associated with wide incisors and short stature. Genetic counselling and offers of investigation of wider family are provided.
Disclosure to insurance companies and beyond
Fear and discrimination towards disclosing genomic disease information to insurance companies have been recurring opinions expressed in survey studies.19 Ownership of personal genomic data may not be as clear-cut as initially imagined, and responsibilities in this respect may soon be explicitly detailed in the small print of medical and even dental insurance policies.
In this context, we should be cautious in any personal desire to explore our own genetic make-up using direct-to-consumer kits. The sequence data generated is not only used to create reports to satisfy our curiosity as the consumer; it is also added to an ever-accumulating genomic database which is used for research into other traits and diseases. While this may make us feel philanthropic about sharing our data to further medical knowledge, commercial companies can also reserve the right to use your genomic data to sell you personally relevant products and services. Although the promise of confidentiality underpins these personalised DNA testing kits, selling en-masse anonymised data to pharmaceutical and insurance companies could be extremely lucrative.
Dental education and counselling
Genetic counselling is an essential step in the genetic testing process. It is offered to patients both before and after having a genetic test.20 The focus of dental genomic education is not to turn members of the dental team into genetic counsellors, but to hone their diagnostic skills, be able to identify abnormal and be capable of taking good family histories and pedigree analyses.21 This set of skills helps select patients suitable for genetic investigation. If a positive diagnosis follows, it is difficult to imagine a highly-trained trained genetic counsellor knowing enough about teeth and dentistry to explain the implications.
The dental team is best placed to provide a clinically relevant interpretation of a genetic dental diagnosis, and to do this some training in human genetics and genomics is needed. This has been proposed for inclusion in the undergraduate dental curriculum, enabling the dental team to consider some of the complex issues surrounding genetic testing, from offering the referral to interpreting any findings.19 It is not currently known how widely this area is covered in UK dental teaching institutions, but a survey of over 50 US dental schools found genetic education to be limited to a minority of responding institutions.22
Conclusion
Enthusiasm towards the concept of genetic testing for dental developmental disorders may become tempered by understanding the profound implications of findings pertinent to dentistry or the discovery of incidental findings relevant to general health. When considering how far we should go in testing for genetic dental disorders, we should bear in mind the old adage that none of us are completely healthy, just under-investigated. Dentists, and the dental research community, need to work with and learn from medical genetics services, particularly with respect to counselling, disclosure and confidentiality. There needs to be further exploration of how dental genetic information is generated, interpreted and communicated.
This brief overview of the potential for dental genomics to become useful in general dental practice aims to stimulate discussion on how this area of dental care should evolve. Genetic testing for dental disorders is already having an impact on care for a small number of patients with rare conditions, and will inevitably become more accessible to the public within NHS dental services. Genomic education should be expanded in undergraduate and postgraduate curricula to reflect this.
References
Gadhia K, McDonald S, Arkutu N, Malik K . Amelogenesis imperfecta: an introduction. Br Dent J 2012; 212: 377–379.
Cobourne M T . Familial human hypodontia – is it all in the genes? Br Dent J 2007; 203: 203–208.
Fleming P S, Xavier G M, DiBiase A T, Cobourne M T . Revisiting the supernumerary: the epidemiological and molecular basis of extra teeth. Br Dent J 2010; 208: 25–30.
Castellsagué X, Alemany L, Quer M et al. HPV Involvement in Head and Neck Cancers: Comprehensive Assessment of Biomarkers in 3: 680 Patients. J Natl Cancer Inst 2016; 108.
de Lima C L, Acevedo A C, Grisl D C, Taba M Jr, Guerra E, de Luca Canto G . Host-derived salivary biomarkers in diagnosing periodontal disease: systematic review and meta-analysis. J Clin Periodontol 2016; 43: 492–502.
von Holstein-Rathlou S, BonDurant L D, Peltekian L et al. FGF21 mediates endocrine control of simple sugar intake and sweet taste preference by the liver. Cell Metab 2016; 9: 335–343.
Kulkarni G V, Chng T, Eny K M, Nielsen D, Wessman C, El-Sohemy A . Association of GLUT2 and TAS1R2 genotypes with risk for dental caries. Caries Res 2013; 47: 219–225.
Hu S, Parker J, Wright J T . Towards unraveling the human tooth transcriptome: the dentome. PLoS One 2015; 10: e0124801.
de la Dure-Molla M, Quentric M, Yamaguti P M et al. Pathognomonic oral profile of Enamel Renal Syndrome (ERS) caused by recessive FAM20A mutations. Orphanet J Rare Dis 2014–14; 9.
Teixeira C S, Santos Felippe M C, Tadeu Felippe W, Silva-Sousa Y T, Sousa-Neto M D . The role of dentists in diagnosing osteogenesis imperfecta in patients with dentinogenesis imperfecta. J Am Dent Assoc 2008; 139: 906–p14.
Whyte M P, Madson K L, Phillips D et al. Asfotase alfa therapy for children with hypophosphatasia. JCI Insight 2016; 1: 1–11.
Carpenter T O, Imel E A, Ruppe M D et al. Randomized trial of the anti-FGF23 antibody KRN23 in Xlinked hypophosphatemia. J Clin Investigation 2014; 124: 1587–1597.
Huttner K . Future developments in XLHED treatment approaches. Am J Med Genet 2014; 164A: 2433–2436.
Lucassen A, Houlston R S . The challenges of genome analysis in the health care setting. Genes 2014; 22: 576–585.
Clarke A . Should families own genetic information? No. Br Med J 2007; 335: 23.
Wright CF, Middleton A, Burton H et al. Policy challenges of clinical genome sequencing. Br Med J 2013; 22: 347.
Green RC, Berg J S, Grody W W et al. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med 2013; 15: 565–574.
Middleton A, Morley KI, Bragin E, Firth H V, Hurles M E, Wright C F, Parker M . No expectation to share incidental findings in genomic research. Lancet 2015; 385: 1289–1290.
Hietala M, Hakonen A, Aro AR, Niemelä P, Peltonen L, Aula P . Attitudes toward genetic testing among the general population and relatives of patients with a severe genetic disease: a survey from Finland. Am J Hum Genet 1995; 56: 1493–1500.
Genetic Alliance UK. 2015. Available at https://www.geneticalliance.org.uk/media/1924/patient-charter-genome-sequencing-what-do-patients-think.pdf (accessed April 2018).
Hart P S, Hart T C . Invited commentary: The need for human genetics and genomics in dental school curricula. Mol Genet Genomic Med 2016; 4: 123–125.
Dudlicek L L, Gettig E A, Etzel K R, Hart T C . Status of Genetics Education in U S Dental Schools. J Dent Ed 2004; 68: 809–818.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harrison, M., Bushell, CJ. & Irving, M. 32 and you – genetic testing for dental disorders. Br Dent J 224, 829–832 (2018). https://doi.org/10.1038/sj.bdj.2018.360
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bdj.2018.360