Key Points
-
Provides an update on oral leukoplakia and proliferative verrucous leukoplakia.
-
Highlights the importance of early recognition and diagnosis of these lesions.
-
Provides an overview of the current investigations and management options.
Abstract
Objectives To provide an overview of the current thinking in terms of the diagnosis and management of oral leukoplakia and proliferative verrucous leukoplakia as relevant to general dental practitioners.
Data sources, data selection, data extraction, data synthesis We searched the MEDLINE Ovid, EMBASE databases and the Cochrane Library, (1990 to 16 April 2017), restricting our search to English language with the following key words: leukoplakia, white patch, proliferative verrucous leukoplakia, precancerous lesion, premalignant lesions, potentially malignant oral conditions and potentially malignant oral disorders. The two authors selected key papers and engaged in collaborative data extraction and synthesis of the selected reference material.
Conclusions General dental practitioners (GDPs) are likely to encounter patients with a known or yet undiagnosed oral leukoplakia in their clinical practice. The diagnosis is clinically based as there are no pathognomonic histopathological features. The definition of leukoplakia has evolved over the years. The importance of recognition and appropriate management relating to this condition is described particularly as it is one of the oral potentially malignant lesions. The inferred increased risk of malignant transformation is well documented however controversy still persists in terms of the appropriate management for these lesions. Proliferative verrucous leukoplakia is a recalcitrant, often widespread and multifocal distinct type of leukoplakia. It is considered to have a high rate of malignant transformation with implications in terms of lifelong monitoring both clinically and histopathologically. A high index of suspicion is important for general dental practitioners in order to identify such lesions that would require onward referral for further investigation and management.
Similar content being viewed by others
Introduction
Oral leukoplakia (OL) is a widely used term to describe white oral mucosal lesions of questionable risk. We aim to describe the correct usage of this term in clinical practice and how it can vary in presentation, investigation and management.
General dental practitioners (GDPs) are likely to encounter patients with a known or yet undiagnosed oral leukoplakia in their clinical practice. The importance of recognition and appropriate management relating to this condition is particularly relevant as it is one of the oral potentially malignant conditions.
Proliferative verrucous leukoplakia (PVL) is a recalcitrant, often widespread and multifocal distinct type of leukoplakia. It is considered to have a high rate of malignant transformation with implications in terms of lifelong close monitoring both clinically and histopathologically.
We searched the MEDLINE Ovid, EMBASE databases and the Cochrane Library, (1990 to 16 April 2017), restricting our search to English Language with the following key words: leukoplakia, white patch, proliferative verrucous leukoplakia, precancerous lesion, premalignant lesions, potentially malignant oral conditions and potentially malignant oral disorders. The two authors selected key papers and engaged in collaborative data extraction and synthesis of the selected reference material.
This overview provides an up-to-date review on the present knowledge of OL and PVL, focused on improving the diagnostic and triage skills of the GDP tasked with managing patients presenting with OL/PVL in practice.
A high index of suspicion is important for GDPs in order to identify such lesions that would require onward referral for further investigation and management.
Oral leukoplakia
Definition of oral leukoplakia
The term OL is defined by the 2005 World Health Organisation (WHO) as 'a white plaque of questionable risk having excluded (other) known diseases or disorders that carry no increased risk for cancer.'1
The first inference arising from this definition is that there are other conditions that may result in a non-removable white patch but which can be differentiated as a specific diagnosis (see Table 1 for examples of such conditions) which should be excluded.
The second important inference regards the potential behaviour of such a lesion in that it innately is associated with an increased risk of development of an oral (or elsewhere within the head and neck) cancer.2
OL is one of the oral potentially malignant disorders. The term 'disorder' is used to reflect the fact that the development of a cancer may also involve sites outside the lesion itself.2
Epidemiology
There is a significant geographical variation in the prevalence of OL influenced by aetiological factors such as betel nut chewing habits in South East Asia. OL is globally more common in male patients, however, the male/female distribution does vary in differing geographical areas. The estimate for prevalence in global terms is 2.6%3 and prevalence in developed countries is around 3%.4
Clinical diagnosis
Establishing a diagnosis of an OL is a clinical process supplemented by clinicopathological correlation. The histopathological features are not pathognomonic and are nonspecific. Histopathological examination is considered mandatory in order to exclude other pathological processes (examples provided in Table 1) that may indicate an alternative definable diagnosis and to determine whether any dysplasia or even frank carcinoma is present within the submitted specimen.5
Clinical types
When a patient presents in a dental practice with an OL, the GDP should assess the patient's medical and social history to include identification of risk factors such as current immunosuppression, history of a previous head and neck carcinoma, tobacco, paan, khat, betel nut habits and alcohol use.
Clinical examination should include detailed inspection of the OL and surrounding tissues noting, in particular, surface architectural changes such as nodularity or verrucous change, areas of erythema within the leukoplakia (erythroleukoplakia), and areas of ulceration. The site and size should be carefully recorded. Palpation of OL is important to detect any underlying firmness which may be present. Clinical photographic records are extremely helpful especially if they accompany a referral to a specialist service as these will prove an invaluable aid to triaging.
The clinical findings can then be interpreted by the GDP in light of his/her knowledge of the condition to inform a discussion with a patient and critical decision-making in terms of management. The key decision points are to continue monitoring in practice, refer onwards as a routine referral or alternatively as an urgent or fast -track referral.
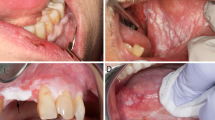
The term homogenous leukoplakia is assigned to a leukoplakia which is typically uniformly white, relatively flat, superficial and with clear demarcated margins (Figs 2 and 3). These lesions are typically free from interspersed areas of fissuring, erythema and without a nodular, verrucous or otherwise irregular surface. The term non-homogenous leukoplakia is conversely applied to leukoplakias with any of the latterly described changes. The margins of non-homogenous leukoplakia also tend to be less well demarcated.1 Non homogenous leukoplakias represent a higher risk lesion as relative to homogenous leukoplakia.6
If there are speckled areas or islands of red patches within the OL then the diagnosis may be revised to a speckled leukoplakia. Erythroplakia of the oral mucosa is a rare but clinically significant diagnosis with a tendency to present in the middle aged and elderly. It is defined as any lesion of the oral mucosa that presents as 'a fiery red patch which cannot be characterised clinically or pathologically as any other definable condition.'7 Similar risk factors to OL apply to the development of erythroplakia.8 Indeed, malignant transformation rates in erythroplakia range from 14%–50% and are the highest out of all the oral potentially malignant disorders.9,10
Aetiology
The majority of OL are considered to develop secondary to smoked tobacco, smokeless tobacco, alcohol and betel quid use. Betel nut chewing habits are a significant aetiological factor in South East Asia driving the increased prevalence of these lesions within this region. Smoking remains the predominant causative factor globally, however, OL does develop in non-smokers too. OLs that arise in the absence of such identifiable risk factors are described as idiopathic leukoplakias and are considered to have an underlying genetic basis for development.11,12
Factors influencing malignant change
While at present there are no reliable markers that can accurately predict individualised malignancy transformation risks of OL, the presence of the following factors are predictive for an increased risk of malignant transformation:13
-
Surface architectural changes such as nodularity or verrucous changes (Fig. 4)
-
Presence of areas of erythema within the leukoplakia (erythroleukoplakia/speckled leukoplakia)
-
Female sex
-
Increased age
-
Idiopathic leukoplakia (in a given population of smokers a higher prevalence of OL is expected, however, if OL has developed in the absence of aetiological factors, then the risk of malignant transformation is higher)
-
Site: floor of mouth, ventrolateral tongue and soft palate involvement14,15,16,17,18,19
-
Dysplasia grade/severity – dysplasia is a pathological term used to describe abnormal epithelial growth characterised by a range of cytologic, maturational, and architectural changes within an epithelium. Dysplasia cannot be diagnosed on a clinical basis and can only be confirmed by histopathological assessment
-
The terms mild, moderate, and severe dysplasia are applied if architectural and cytologic atypia involve less than a third, two thirds or the full extent of the epithelium. A histopathological diagnosis of carcinoma in situ is assigned when dysplasia involves the entire extent of the epithelium full thickness but within the confines of the basement membrane. The increased risk of malignant transformation rates in association with dysplasia is well documented in the literature, with transformation rates ranging from 5%–36%. The risk is considered to increase with increased grade/severity of dysplasia and hence it is critical information in assessing the risk of an OL17,21,22,23
-
A history of a previous head and neck carcinoma13
-
Candidal infection in OL has been postulated as a risk factor in terms of malignant transformation. The underlying theory centres on the ability of certain candidal strains forming nitrosamines which are known carcinogens.20 When one considers that such reported infection occurs in 27–81% of the cases, appropriate antimicrobial therapy should be considered as part of the management strategy in cases where such co-infection is identified.24 Chronic hyperplastic candidosis is a form of oral candidiosis that can be referred to as candidal leukoplakia. It typically involves the commissures, is often present in smokers and the diagnosis is confirmed by the demonstration of fungal hyphae on histopathological examination along with clinicopathological correlation (Fig. 5).
Human papillomavirus and OL
There has been much reported on the link between human papillomavirus (HPV) and oral squamous cell carcinoma (OSCC) but it is less evident as to the association between HPV and OL. There is little evidence to support a causal link between oral leukoplakia and HPV.25 A meta-analysis of 94 studies analysing 4,670 samples was carried out which found that HPV was between 2–3 times more likely to be found in pre-malignant lesions and 4.7 times more likely in OSCC than in normal mucosa.26
Transformation rates of OL
One of the most commonly asked questions by patients consequent to being informed of a diagnosis of an OL is 'what is the risk of this lesion transforming into a cancer?'
The generic answer often provided by clinicians is that the risk is patient specific and cannot be predicted with accuracy. The annual transformation rate (ATR) of OL is a helpful generic guide to quote as a starting point. This rate is presented in several studies and is obtained through a process of division of the number of patients who experienced a malignant transformation in their OL by the entire number of patients followed up in that particular study in a one-year period. Unfortunately, not all relevant studies include time frame-based data allowing ATR to be estimated. From current data available, it is considered that the annual transformation rate of OL is around 2–3%.13
Factors outlined above such as site, grade of dysplasia, idiopathic nature and non-homogenous leukoplakia would result in patient-related specific risks that would need to be discussed with the patient concerned.27,28,29,30
Diagnosis, management and interventions
Most cases of OL should be referred to secondary care for assessment as a routine referral, however, in the presence of signs such as speckling, induration, swelling and ulceration, additional clinical concern would justify escalating to a two week wait referral. Where doubt exists with regard to the appropriate referral urgency, GDPs are advised to include all relevant factors in the referral and, additionally, send high quality clinical photographs which will help the triaging clinician.
Current guidance for GDPs for the referral of lesions which are suspected as oral cancer were included in an updated NICE guideline (June 2015). The guidance states that 'a red or red and white patch in the oral cavity consistent with erythroplakia or erythroleukoplakia' should be referred on a defined suspected cancer pathway to be seen within two weeks.32
Patients naturally enquire as to whether these lesions will resolve spontaneously and indeed a minority of OL will do so.14 Patients also enquire about active treatment strategies that could potentially resolve the OL or indeed reduce the risk of malignant transformation. Any such discussion would need to be informed by clinical judgement and the underlying evidence base to allow appropriate shared care decision-making with the concerned patient.
Biopsy and histopathological examination is mandatory to exclude other conditions. It is not expected that these investigations are carried out in general dental practice and we would advise that such investigations are left to secondary care clinicians. Selection of the biopsy site and appropriate biopsy technique are important factors in this process, enabling the histopathologist to report their findings. The histopathological examination will help to exclude other pathological processes and is particularly important to determine the presence of dysplasia or indeed oral squamous cell carcinoma.
Medical management
There is currently no high-quality evidence to support systemic treatment-based medication or indeed alternative medicinal drugs as an intervention for OL.30,31,33,35
Hence medical management options are not routinely discussed in oral medicine clinics within the UK.
Surgical management
Surgical excision of OL is a treatment option to be considered and discussed with patients especially where the risk of malignant transformation is deemed to be significant. The key clinical question is whether the surgical morbidity consequent to excision of an oral leukoplakia can be clinically justified.
A systematic meta-analysis by Mehanna et al. did report a reduction in malignancy transformation rates of oral potentially malignant disorders (including OL), especially where moderate/severe dysplasia was present which was managed with surgical excision.29
However, a recent Cochrane review considering interventions for OL, concluded that there is a lack of high quality evidence to underpin routine surgical management of leukoplakias such as conventional scalpel excision, laser excision, photodynamic therapy or cryosurgery.35 This systematic review identified that there were no randomised controlled studies assessing surgical intervention against no treatments or placebo available.35 This finding is further supported by other retrospective studies.36,37
The lack of incontrovertible evidence to inform surgical management of OL results in a clinical dilemma encountered in specialist clinics on a daily basis. Clinicians should ensure that patients understand that the evidence shortfall may simply be a reflection of the ethical challenges involved in conducting large scale studies of sufficient power with adequate long term clinical follow up.
The annual recurrence rate of OL following surgical excision is considered to be around 5%–10% and hence patients considering this option need to be appraised of this risk. However, this is a global estimate and the inherent risk of an OL can vary significantly which would hence need to be factored into the decision-making process.38
The advantages of surgical excision include the potential benefits of providing an entire lesion for histopathological assessment. Studies have shown that OLs, excised and histopathologically examined, have contained foci of oral squamous cell carcinoma in 7–12% of cases.14,39
The process of carcinogenesis within the oral cavity results from an accumulation of genetic defects. These changes themselves may be more advanced in areas which are phenotypically normal, hence outside the lesional tissue. This so called concept of 'field change' adds complexity to surgical considerations in terms of selection of whether to remove a leukoplakia and also whether to include adjacent phenotypically normal tissue.14,40,41
Some authors have suggested that reducing the risk of recurrence and in turn of malignant transformation would require excision of all areas which contain epithelial abnormalities beyond the area of the leukoplakia itself.38
Ultimately, the role of the clinician is to guide and facilitate the decision-making process of the patient, helping them understand both the potential risks and benefits and placing the current lack of high grade evidence within the clinical context specific to that patient. Within this context multidisciplinary clinics with both oral medicine and surgical input may provide an appropriate environment for complex cases where the decision is not straightforward.33,34
The potential future development of specific predictive tools and underpinning evidence to inform management will hopefully enable clinicians to guide the patients accordingly in the future.
Modification of risk factors
Patients are routinely encouraged to reduce/stop tobacco use and alcohol consumption as part of their clinical management. Patients using snuff, betel or areca nut are similarly encouraged to stop. The GDP has an important role to play as a healthcare worker who often has regular contact with the patient on an ongoing basis within this context.
The safety of vaping and electronic nicotine delivery systems is yet undetermined and the devices are unregulated, however, it is generally considered to be potentially safer as compared to tobacco use although further research is required into the safety and range of products available.42 Smoking cessation can be followed by resolution of an OL in some patients, inevitably with a delay of several months. Cessation of smoking habits considerably reduces the risk of developing cancer after surgical treatment of oral potentially malignant lesions.43
Monitoring
There is no evidence of the efficacy of long-term clinical follow up in patients with an OL in terms of prevention of oral cancer development.13 Despite the lack of evidence, close surveillance is considered good practice and patients often prefer this strategy.
While there is no specific guidance, 3–6 monthly follow-up appointments are the norm based on anticipated risk and patient preference. While most of this follow up process within the UK tends to occur in secondary care setting, GDPs are likely to increasingly be called upon to participate in the monitoring of these lesions as part of a comprehensive management plan within a managed clinical network. Consequently, GDPs should be familiar with signs or changes that raise concerns and respond accordingly through prompt referral.
There has been much research looking at salivary biomarkers as a method of early oral cancer detection, this is a promising field but no single biomarker is yet to be used routinely in clinical detection for OL lesions.44
Toluidine blue, brush cytology, tissue reflectance and autofluorescence have all been evaluated as diagnostic aids for oral cancer detection in suspicious lesions, however, these have not been proven to improve detection of oral cancer beyond conventional oral examination.45,46,47
Proliferative verrucous leukoplakia
Proliferative verrucous leukoplakia (PVL) is a rare, recalcitrant, often widespread and multifocal distinct type of leukoplakia. PVL was first described by Hansen et al., in 1985 who presented 30 cases of OLs which were described as starting as a simple hyperkeratosis and then spreading to become multifocal.48
The WHO described PVL in 2005 as 'rare but distinctive high-risk clinical form of oral precursor lesion.'49,50 PVL is therefore generally thought of as being a distinct and particularly aggressive type of OL with a high rate of recurrence.51
Clinical characteristics and features
The low prevalence of PVL means data on clinical features is limited mainly to case reports, case series and a few case control studies although there has been a couple of recent systematic reviews.51,52 The clinical features include the findings that PVL is more commonly found in elderly female patients49 and non-smokers. It is thought to typically progress from one or more areas of homogenous leukoplakia to become multifocal areas of leukoplakia with exophytic, wart-like lesions that enlarge and spread (Fig. 6).
A recent systematic review pooled the data for the site distribution of PVL with the most frequently involved site being the gingivae (62.7%), followed by the buccal mucosa (59.8%) and the tongue (49.1%).48,51
In order to make the diagnosis of PVL major and minor criteria have been suggested in 2010 by Cerero-Lapiedra et al.53 which have been more recently modified to four diagnostic criteria.52 These include; the presence of verrucous or wart-like leukoplakia involving two or more sub-sites, when adding all the involved sites the minimum size of the lesions should be at least three cm, at least five years of disease evolution and the availability of at least one biopsy ruling out a verrucous carcinoma or squamous cell carcinoma.54
Aetiology
Smoking and alcohol consumption are traditionally thought of as risk factors in OL and oral squamous cell carcinomas (OSCC) but in PVL there is no clear link with these. In a comparison between PVL and OL there was no significant difference between the two groups in the demographic factors which included alcohol and smoking.55
There is a variation in HPV prevalence reported in PVL cases with the reported range being from 0%56 to 88%56,57 detection of HPV DNA although both of these results are from small numbers of cases (cases N = 13 or less). In a larger case series (N = 58) the HPV positive cases were found to be 24%, with no significant difference found between the PVL group and an OL group (25% HPV positive).55 The role of microorganisms such as C. albicans in PVL has not been fully established.58 DNA ploidy is a test that has been suggested to be able to be used on cells to predict the behaviour of the DNA and possible prediction of malignant transformation. Gouvea et al.59 found that 95% of cases analysed showed aneuploidy and abnormal DNA was seen in even the more benign appearing lesions.
Diagnosis and management
There is no single histopathological characteristic that defines a diagnosis of PVL and usually diagnosis is based on the combination of the histological and clinical findings.52 Often multiple biopsies are carried out of any clinically suspicious areas. The histology can show a range of features from hyperkeratosis in the early stages, epithelial hyperplasia and atypia which can then progress onto verrucous hyperplasia, verrucous carcinoma and eventually to squamous cell carcinoma (SCC). This progression and different stages in PVL indicates the need for multiple and repeat biopsies over time to monitor for dysplasia and possible progression to SCC.52
A review of the literature deems that overall PVL can be resistant to different treatment modalities including surgery, laser ablation, retinoids, photodynamic therapy and chemotherapy. No treatment appears effective in reducing the risk of malignant transformation.58 Even with the lack of evidence, surgery tends to be the most frequently used intervention for PVL.52 A recent literature review, however, found that there was a mean recurrence rate of 85% for all treatment modalities used to manage this condition.58
PVL has a higher rate of malignant transformation than OL. PVL can develop both OSCC and oral verrucous carcinomas (OVC). In one large case series (N = 55) of patients with PVL the demographics of the patients who went on to develop PVL were more frequently female and non-smokers.49
The transformation rate in PVL varies between case series. In a systematic review carried out in 2014, 12 studies were merged which reported the overall transformation rate was 56.2%, but when considering an average follow-up period of 7.4 years the transformation rate was higher at 60.7%.51 A more recent systematic review in 2015 put the transformation rate higher at 63.9% in a subgroup analysis of 277 patients with PVL where about 40% of patients died of the disease.52
Conclusions
This overview has aimed to highlight what is known about OL, the variety of presentations and multiple aetiologies.
While it is well publicised that OL has an increased risk of malignant potential there is still debate around the gold standard for investigating and management of these lesions. This is particularly true of the rarer types of OL as demonstrated in PVL where there is still no ideal management option and the patients are at high risk of development of malignant lesions. There is a recognised need for further research and long term randomised controlled trials, however, this is fraught with difficulty in terms of the exact definition, remit and extent of these lesions.
GDPs are likely to encounter patients with a known or yet undiagnosed OL or PVL in their clinical practice. The importance of recognition of these lesions by GDPs and appropriate management including appropriate onward referral has been highlighted. GDPs should appreciate that a subtle change in the clinical presentation may represent the development of a malignancy in the lesion itself. Furthermore, both OL and PVL can harbour areas of occult malignancy. GDPs armed with this knowledge should be able to include all relevant factors in any referral to allow appropriate triage by secondary practitioners.
Understanding the current concepts around OL and PVL will place the GDP in a better position to decide whether to refer and what type of referral is required. The patient being referred will inevitably have questions about rationale for such a referral, risks of such lesions and the likely process that will be followed in secondary care. GDPs will also potentially have responsibility for the monitoring of these lesions as part of a comprehensive management plan.
In summary, this review has aimed to increase the knowledge of GDPs regarding OL and PVL, to inform their critical decision-making and diagnosis, and to allow them to discuss their decision confidently with their patients.
References
Warnakulasuriya S, Johnson N W, Van Der Waal I . Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med 2007; 36: 575–580.
van der Waal I . Oral leukoplakia, the ongoing discussion on definition and terminology. Med Oral Patol Oral Cir Bucal 2015; 20: e685–e692.
Petti S . Pooled estimate of world leukoplakia prevalence: a systematic review. Oral Oncol 2003; 39: 770–780.
van der Waal I . Potentially malignant disorders of the oral and oropharyngeal mucosa; terminology, classification and present concepts of management. Oral Oncol 2009; 45: 317–23.
Napier S S, Speight P M . Natural history of potentially malignant oral lesions and conditions: an overview of the literature. J Oral Pathol Med 2008; 37: 1–10.
van der Waal I, Axéll T . Oral leukoplakia: a proposal for uniform reporting. Oral Oncol 2002; 38: 521–526.
Pindborg J J, Reichart P A, Smith C J, van der Waal I . Histological Classification of Cancer and Precancer of the Oral Mucosa. In Histological Typing of Cancer and Precancer of the Oral Mucosa. pp. 9–10. Heidelberg, Gemany: Springer, 1997.
Hashibe M, Mathew B, Kuruvilla B et al. Chewing tobacco, alcohol, and the risk of erythroplakia. Cancer Epidemiol Biomarkers Prev 2000; 9: 639–645.
Reichart P A, Philipsen H P . Oral erythroplakiaa review. Oral Oncol 2005; 41: 551–561.
Bouquot J E, Ephros H . Erythroplakia: the dangerous red mucosa. Pract Periodontics Aesthet Dent 1995; 7: 59–67; quiz 68.
Khan Z, Khan S, Christianson L, Rehman S, Ekwunife O, Samkange-Zeeb F. Smokeless tobacco and oral potentially malignant disorders in South Asia: a systematic review and meta-analysis. Nicotine Tob Res 2016; Available at https://academic.oup.com/ntr/article-abstract/doi/10.1093/ntr/ntw310/2632180/Smokeless-Tobacco-and-Oral-Potentially-Malignant?redirectedFrom=fulltext (accessed October 2017).
van der Waal I . Potentially malignant disorders of the oral and oropharyngeal mucosa; present concepts of management. Oral Oncol 2010; 46: 423–425.
van der Waal I . Oral potentially malignant disorders: is malignant transformation predictable and preventable? Med Oral Patol Oral Cir Bucal 2014; 19: e386–e390.
Holmstrup P, Vedtofte P, Reibel J, Stoltze K . Long-term treatment outcome of oral premalignant lesions. Oral Oncol 2006; 42: 461–474.
Schepman KP, van der Meij E H, Smeele LE, van der Waal I . Prevalence study of oral white lesions with special reference to a new definition of oral leucoplakia. Eur J Cancer B Oral Oncol 1996; 32B: 416–419.
Jr S S, Silverman S Jr, Gorsky M, Lozada F, Dds, Ms. Oral leukoplakia and malignant transformation. A follow-up study of 257 patients. Cancer 1984; 53: 563–568.
Liu W, Shi LJ, Wu L et al. Oral cancer development in patients with leukoplakiaclinicopathological factors affecting outcome. PLoS One 2012 Apr 13; 7: e34773.
Warnakulasuriya S, Kovacevic T, Madden P et al. Factors predicting malignant transformation in oral potentially malignant disorders among patients accrued over a 10-year period in South East England. J Oral Pathol Med 2011; 40: 677–683.
Warnakulasuriya S, Ariyawardana A . Malignant transformation of oral leukoplakia: a systematic review of observational studies. J Oral Pathol Med 2016; 45: 155–166.
Sanjaya P R, Gokul S, Gururaj Patil B, Raju R . Candida in oral pre-cancer and oral cancer. Med Hypotheses 2011; 77: 1125–1128.
Cowan C G, Gregg T A, Napier S S, McKenna S M, Kee F . Potentially malignant oral lesions in northern Ireland: a 20-year population-based perspective of malignant transformation. Oral Dis 2001; 7: 18–24.
McAlinden R L, Maxwell P, Napier S et al. Oral and Maxillofacial Pathology: Bcl2 expression in sequential biopsies of potentially malignant oral mucosal lesions assessed by immunocytochemistry. Oral Dis 2008; 6: 318–326.
Lumerman H, Freedman P, Kerpel S . Oral epithelial dysplasia and the development of invasive squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1995; 79: 321–329.
Rindum J L, Stenderup A, Holmstrup P . Identification of Candida albicans types related to healthy and pathological oral mucosa. J Oral Pathol Med 1994; 23: 406–412.
Feller L, Lemmer J . Oral Leukoplakia as It Relates to HPV Infection: A Review. Int J Dent 2012; 2012: 1–7.
Miller C S, Johnstone B M . Human papillomavirus as a risk factor for oral squamous cell carcinoma: a meta-analysis, 1982–1997. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001; 91: 622–635.
Scheifele C, Reichart P A . Is there a natural limit of the transformation rate of oral leukoplakia? Oral Oncol 2003; 39: 470–475.
Pentenero M, Giaretti W, Navone R et al. Evidence for a possible anatomical subsite-mediated effect of tobacco in oral potentially malignant disorders and carcinoma. J Oral Pathol Med 2011; 40: 214–217.
Mehanna H M, Rattay T, Smith J, McConkey C C . Treatment and follow-up of oral dysplasia – a systematic review and meta-analysis. Head Neck 2009; 31: 1600–1609.
Ho M W, Risk J M, Woolgar J A et al. The clinical determinants of malignant transformation in oral epithelial dysplasia. Oral Oncol 2012; 48: 969–976.
William W N . Oral premalignant lesions. Curr Opin Oncol 2012; 24: 205–210.
NICE. Suspected cancer: recognition and referral. Available at https://www.nice.org.uk/guidance/ng12/chapter/Introduction#head-and-neck-cancers (accessed May 2017).
Field E A, McCarthy C E, Ho M W et al. The management of oral epithelial dysplasia: The Liverpool algorithm. Oral Oncol 2015; 51: 883–887.
Holmstrup P . Can we prevent malignancy by treating premalignant lesions? Oral Oncol 2009; 45: 549–550.
Lodi G, Franchini R, Warnakulasuriya S et al. Interventions for treating oral leukoplakia to prevent oral cancer. Cochrane Database Syst Rev 2016; 7: CD001829.
Arnaoutakis D, Bishop J, Westra W, Califano J A . Recurrence patterns and management of oral cavity premalignant lesions. Oral Oncol 2013; 49: 814–817.
Arduino P G, Surace A, Carbone M et al. Outcome of oral dysplasia: a retrospective hospital-based study of 207 patients with a long follow-up. J Oral Pathol Med 2009; 38: 540–544.
Kuribayashi Y, Tsushima F, Sato M, Morita KI, Omura K . Recurrence patterns of oral leukoplakia after curative surgical resection: important factors that predict the risk of recurrence and malignancy. J Oral Pathol Med 2012; 41: 682–688.
Thomson P J, Goodson M L, Cocks K, Turner J E . Interventional laser surgery for oral potentially malignant disorders: a longitudinal patient cohort study. Int J Oral Maxillofac Surg 2016 46: 337–342.
Gomes C C, Fonseca-Silva T, Galvão C F, Friedman E, De Marco L, Gomez R S . Interand intra-lesional molecular heterogeneity of oral leukoplakia. Oral Oncol 2015; 51: 178–181.
Braakhuis B J M, Leemans C R, Visser O . Incidence and survival trends of head and neck squamous cell carcinoma in the Netherlands between 1989 and 2011. Oral Oncol 2014; 50: 670–675.
Harrell P T, Simmons V N, Correa J B, Padhya T A, Brandon T H . Electronic Nicotine Delivery Systems ('E-cigarettes'): Review of Safety and Smoking Cessation Efficacy. Otolaryngol Head Neck Surg 2014; 151: 381–393.
Vladimirov B S, Schiodt M . The effect of quitting smoking on the risk of unfavourable events after surgical treatment of oral potentially malignant lesions. Int J Oral Maxillofac Surg 2009; 38: 1188–1193.
Cheng YS L, Rees T, Wright J . A review of research on salivary biomarkers for oral cancer detection. Clin Transl Med 2014; 3: 3.
Lingen M W, Kalmar J R, Karrison T, Speight P M . Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol 2008; 44: 10–22.
Seoane Lestón J, Diz Dios P . Diagnostic clinical aids in oral cancer. Oral Oncol 2010; 46: 418–422.
Fedele S . Diagnostic aids in the screening of oral cancer. Head Neck Oncol 2009; 1: 5.
Hansen L S, Olson J A, Silverman S Jr . Proliferative verrucous leukoplakia. A long-term study of thirty patients. Oral Surg Oral Med Oral Pathol 1985; 60: 285–298.
Bagan J V, Jiménez-Soriano Y, Diaz-Fernandez J M et al. Malignant transformation of proliferative verrucous leukoplakia to oral squamous cell carcinoma: a series of 55 cases. Oral Oncol 2011; 47: 732–735.
World Health Organisation. Pathology and Genetics of Head and Neck Tumours. IARC, 2005.
Pentenero M, Meleti M, Vescovi P, Gandolfo S . Oral proliferative verrucous leucoplakia: are there particular features for such an ambiguous entity? A systematic review. Br J Dermatol 2014; 170: 1039–1047.
Abadie W M, Partington E J, Fowler C B, Schmalbach C E . Optimal Management of Proliferative Verrucous Leukoplakia: A Systematic Review of the Literature. Otolaryngol Head Neck Surg 2015; 153: 504–511.
Cerero-Lapiedra R, Baladé-Martínez D, Moreno-López LA, Esparza-Gómez G, Bagán J V . Proliferative verrucous leukoplakia: a proposal for diagnostic criteria. Med Oral Patol Oral Cir Bucal 2010; 15: e839–e845.
Carrard V C, Brouns EREA, van der Waal I . Proliferative verrucous leukoplakia; a critical appraisal of the diagnostic criteria. Med Oral Patol Oral Cir Bucal 2013; 18: e411–e413.
Campisi G, Giovannelli L, Ammatuna P et al. Proliferative verrucous vs conventional leukoplakia: no significantly increased risk of HPV infection. Oral Oncol 2004; 40: 835–840.
Bagan J V, Jimenez Y, Murillo J, Gavaldá C, Poveda R, Scully C et al. Lack of association between proliferative verrucous leukoplakia and human papillomavirus infection. J Oral Maxillofac Surg 2007; 65: 46–49.
Palefsky J M, Silverman S Jr, Abdel-Salaam M, Daniels T E, Greenspan J S . Association between proliferative verrucous leukoplakia and infection with human papillomavirus type 16. J Oral Pathol Med 1995; 24: 193–197.
Capella D L, Gonçalves J M, Abrantes A A A, Grando L J, Daniel F I . Proliferative verrucous leukoplakia: diagnosis, management and current advances. Braz J Otorhinolaryngol 2017; 83: 585–593.
Gouvêa A F, Santos Silva A R, Speight P M et al. High incidence of DNA ploidy abnormalities and increased Mcm2 expression may predict malignant change in oral proliferative verrucous leukoplakia. Histopathology 2013; 62: 551–562.
Author information
Authors and Affiliations
Corresponding author
Additional information
Refereed Paper
Rights and permissions
About this article
Cite this article
Staines, K., Rogers, H. Oral leukoplakia and proliferative verrucous leukoplakia: a review for dental practitioners. Br Dent J 223, 655–661 (2017). https://doi.org/10.1038/sj.bdj.2017.881
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bdj.2017.881
This article is cited by
-
Laser therapy decreases oral leukoplakia recurrence and boosts patient comfort: a network meta-analysis and systematic review
BMC Oral Health (2024)
-
Proliferative verrucous leukoplakia: a general dental practitioner-focused clinical review
British Dental Journal (2024)
-
Improvement in the risk assessment of oral leukoplakia through morphology-related copy number analysis
Science China Life Sciences (2021)