Key Points
-
This paper highlights some of the risks of causing nerve injury during planning, preparation and placement of mandibular implants.
-
Highlights potential pitfalls and problems.
-
Provides tips on how to prevent these implant related trigeminal injuries.
Abstract
Background The incidence of implant-related inferior alveolar nerve injuries (IANI) is steadily increasing within the UK population.
Aims This study prospectively reviewed thirty cases (35% male; 65% female) of implant-related IANI seen in a specialist nerve injury clinic.
Methods Neurosensory examinations were carried out to ascertain a quantifiable rating of the perception, pain profiling and functional difficulties. Data were analysed using SPSS software.
Results Patients were aware of signing consent forms for the surgery in 11 cases and 8 of those felt they were not explicitly warned about nerve injury. Over 70% of patients were referred after six months post injury. Implant surgery planning involved intra-oral films only (30%), CBCT (10%), dental pantomograph (50%) and long cone peri-apical radiographs (48%). However, no radiographic evidence pre- or postoperatively was provided by the referring practitioner in 15% of cases. Intra-operative problems included bleeding and neurological symptoms. Proximity of the implant bed or implant to the inferior alveolar canal was evident radiographically. This showed contact with roof inferior alveolar nerve canal in 44% of cases, protrusion into the canal in 20% of cases, crossing of the canal in 20% cases and distance in one case, presumed to be due to local anaesthetic injury. All patients presented with a demonstrable neuropathy, which included neuropathic pain (50%) that interfered with speaking, kissing and socialising.
Conclusions Consent, preoperative planning and appropriate referral were inadequate in provision of mandibular implants in this patient group. Recommendations have been proposed to improve practice and possible novel strategies are suggested for the prevention and improved management of these complications.
Similar content being viewed by others
Introduction
Trigeminal nerve injury is a frequent problematic consequence of dental surgical procedures with major medico-legal implications.1 The incidence of lingual nerve injury has remained static in the UK over the last 30 years, however, the incidence of inferior alveolar nerve injury has increased as a result of implant surgery and endodontic therapy.2
Altered sensation and pain in the orofacial region may interfere with speaking, eating, kissing, shaving, applying make-up, tooth brushing and drinking. In fact, just about every social interaction we take for granted.3 These injuries therefore have a significant effect on the patient's quality of life and the iatrogenesis of these injuries may lead to further significant psychological effects.4
The inferior alveolar nerve (IAN) is at a greater risk from injury compared to the lingual nerve (LN), as it is contained within a bony canal, predisposing it to ischaemic trauma and a higher incidence of permanent damage. Implant surgery may cause trauma to the nerve within the canal and local anaesthetic injections may cause injuries, though neuropathy related to block injections is usually temporary but can occasionally persist and become permanent.
There are rare reports of resolution of implant related IAN neuropathies at over four years,5 but these do not comply with normal reports of peripheral sensory nerve injuries.6 Another study recommends referral of injuries after six months7 but this may be too late for many other peripheral sensory nerve injuries. We now understand that after three months, permanent central and peripheral changes occur within the central nervous system subsequent to injury, which are unlikely to respond to surgical intervention.8
The incidence of implant related IANI's varies from 0-33.2% (Table 1).7,9,10,11,12,13,14,15,16 Bone graft harvesting is also associated with IANI's. Appropriate planning, assessment and training should be undertaken in order to minimise injury.17 Avoidance of nerve injury is sometimes attempted by using techniques including nerve lateralisation and alveolar distraction, however, these procedures are themselves high-risk, and may be more likely to result in injury. Interestingly, 25% of 110 edentulous patients included in a randomised controlled clinical trial presented with a degree of altered IAN function before implant placement, perhaps suggesting the need for pre-operative neurosensory evaluation.12
The aim of this report was to investigate the presentation of patients with iatrogenic implant related nerve injury, and to identify potential issues in a prospective cohort of patients seen on a specialist pain clinic, hoping to form recommendations to reduce injury related to implant surgery.
Methods
Subjects
A total of 287 patients with trigeminal nerve injuries collected over three years were consulted at the Dental Institute in King's College Hospital, London. Thirty patients presented with neuropathy in relation to implant treatment. A simple clinical neurosensory examination was applied to enable a quantified rating of the perception, pain profiling and functional difficulties.
Assessment methods
A detailed history was initially taken including: use of local anaesthesia, type of implant, pain during preparation and placement, intra-operative bleeding, the use of radiographs and the distance from IAN canal postoperatively. The patients' self-assessment of neurosensory function in terms of reduced function (hypoesthesia, anaesthesia, ageusia) and neurogenic discomfort (paraesthesia, dysaesthesia, allodynia, dysgeusia) were recorded. Provoking factors and pain characteristics and the interference with daily function were explored. All patients completed psychometric assessments and were assessed by a liaison psychiatrist if it was felt that the patient was struggling to cope with developments.
The clinical examination was based on recommendations by Robinson et al.18 and the neurosensory functions were clarified by the same observer (TR) who carried out a series of previously standardised tests.16,19,20 These tests involved utilising a similar kit of instruments that allowed mapping neuropathic area percentage of dermatome (extra-oral and intra-oral) and assessment of the subjective function score.
The percentage neuropathic area, defined as the percentage area of the intra-oral mucosa and extra-oral skin affected by the injury with altered sensation, was mapped by running closed forceps gently over the surface from unaffected area to the injured zone, mapping points when the patient acknowledged change in sensation. The patients' mean subjective function (SF) was then determined by initially stimulating the opposite side to the injury with a no. 15 filament (equivalent to 50 g/mm2 pressure). The patient was asked to imagine that this sensation was equivalent to 10/10 and no touching in the area being regarded as 0/10. The injured side was then stimulated in the same way and the patient was asked to score their sensory experience (0-10) relative to the uninjured side. Hypersensitivity and possible allodynia to touch and/or thermal stimuli was indicated by an SF value greater than ten.
These tests were followed by assessing light touch, pin prick, sharp blunt discrimination, brush stroke direction and two point discrimination thresholds. Pain levels were obtained using a visual analogue scale (VAS) (score 0-10), at rest and after mechanical and cold stimulation using ethyl chloride. Functionality was assessed by asking specific questions about interference of their symptoms with their daily activity.
Management of patient's symptoms following the referral to us at King's College Hospital Dental Institute included immediate surgical intervention when possible by removing the associated implant, medical management for pain (systemic drugs including anti-depressants or anti-epileptics or topical lidocaine patches (5%) and Botox injections), counselling and more recently introduced cognitive behavioural therapy.
Statistics
All data were analysed using the SPSS statistical programme. Side differences between the healthy and the injured side were tested with students' t-test and a chi-square test was applied for non-parametric testing of frequencies. The value of p ≤0.05 was chosen as the level of statistical significance. Appropriate correlations were also carried out between certain data sets.
Results
The gender distribution of the patients was 35% male and 65% female with a mean age of 50.6 years (range = 26-80 years). The patients were aware of signing consent forms for the implant surgery in 11 cases and of those, 8 felt they were not explicitly warned about nerve injury. Sixty-four percent of patients did not recall providing written consent. Seventy percent of patients were referred to King's College Hospital Dental Institute more than six months after surgery (Table 2) and only a few were referred or treated immediately post surgery.
Pre-operative radiography was undertaken in most cases by the referring clinician/dentist, however, over a third of patients had intra-oral films only on presentation. The radiography undertaken included cone-beam computed tomography (CBCT) in three cases (10%), dental pantomography in 50% of cases and long cone peri-apical radiographs in 48%. No radiographic evidence pre- or postoperatively was provided by the referring practitioner in 15% of cases.
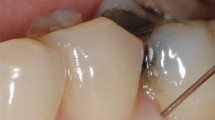
The most common implant position associated with IANI was the lower left second premolar (33% of cases) followed by lower left and right first molar (both 25% of cases) and lower right first premolar in 12% of cases. Figure 1 illustrates placement of an implant in close proximity to the mental loop. Length of the implants ranged from 5-13 mm. Proximity of the implants or the preparation bed to the inferior dental canal was seen in 25 of the 30 cases. In four cases the implant was placed over 18 months before presentation and it could be presumed that bony infill may have taken place. In one case, referred 3 months after surgery, the implants were significantly distant to the IAN canal and it was felt that this injury was related to an articaine mental block. In 13 cases, the roof of the canal appeared compromised (Table 2) and in 12 cases the implant protruded into the IAN canal. The implant crossed the canal in six of these cases, and in one case the patient had bilateral implants invading the right and left IAN canal.
Articaine was used for operative analgesia in 33% of cases, lidocaine was used for 24% of cases and the type of local anaesthetic (LA) was unknown in 20% of cases. A combination of sedation and LA was used for 5% of cases. Intra-operative problems were experienced by 70% of patients. Interruption of the procedure to manage bleeding during drilling of the implant bed occurred in 11 patients. Four patients experienced severe intra-operative bleeding and some form of pain during placement. Seven patients described specific neural stimulation symptoms during implant placement; predominantly pain, but also sensations including tingling, hot water sensation on the chin and shooting pain. Referring clinicians reported sudden 'give' (one case), displacement and re-preparation of implant bed (one case) and implant 'back up' (reversal of implant away from the nerve) in another case.
Features of neuropathy
Permanent IAN neuropathy was sustained in 27 patients. Three patients achieved resolution of neuropathy after removal of the implant within 30 hours of placement. The mean neuropathic areas affecting the extra-oral dermatome and intra-oral dermatome were similar, at 58.1% (range 0.05%-100%; SE 6.4) and 50.8% (range 10-100%; SE 3.8), respectively.
Over 50% of patients suffered from constant pain and or discomfort (Fig. 2). Neuropathic pain was a significant feature and resulted in elicited mechanical or thermal allodynia in 30% of cases. Paraesthesia was the main feature for 47% of cases. Forty percent of patients complained of numbness (or anaesthesia), of which four cases had anaesthesia with pain and four cases had anaesthesia with paraesthesia. Eleven patients reported mechanical allodynia and 14 mechanical hyperalgesia. Seven patients demonstrated cold allodynia and four patients cold hyperalgesia. One patient demonstrated cold and heat induced pain.
Mechanosensory function was significantly reduced in most cases (24/30 cases; Table 2). Subjective function was also reduced in most cases; however, the mean values may not truly reflect this (mean maximum = 9.7 [1-25]; mean minimum = 3.2 [1-5]) as many patients suffered from elicited dysaesthesia, pain and paraesthesia on mechanical stimulation, thus complicating the outcome with elevated scores.
Daily disability and functional problems caused by the neuropathy and pain were extensive in this patient cohort (Fig. 3). Kissing was reduced in pleasure in 54% of the patients and speech was affected in 46% of patients. Over 30% of patients also had problems with eating, drinking and brushing their teeth due to pain. Recurrent lip biting (23%), dribbling (33%) and psychological problems were reported by 30% of patients. Alarmingly, this included four patients with diagnosed depression and two with significant depression and suicidal thoughts, both affective disorders not present pre-operatively. The occurrence of dribbling and retained food on chin when eating in public often resulted in social embarrassment and consequently interfered with their ability to socialise.
Management of the patients
Adjunctive care (Table 3) before referral occurred in several patients and this included non-steroidal anti-inflammatory drugs and steroids in two patients and referral to other dentists and specialists. Three patients who were referred to us and had their implants removed within 30 hours demonstrated resolution of their neuropathy over several weeks as a direct result. Ten patients had their implants removed between three days and six months post implant placement, before their referral to King's College Hospital Dental Institute (KCHDI) and none of these patients demonstrated resolution of neuropathy. All patients were consulted and reassured at their consultation appointment at KCHDI and offered an explanation of their symptoms, of which 13 patients were discharged after 2-4 consultations. Other patients were treated with medications including tricyclic antidepressants, pregabalin, topical 5% lidocaine patches (Versatis), benzoncaine topical LA cream and LA with Botox injections. Cognitive behavioural therapy (CBT) was of benefit to eight patients. CBT, which is a psychotherapeutic approach, helped manage patients to better deal with their coping strategies by talking through their problems and following a goal-orientated, systematic procedure.
Discussion
Patient presentation
Injuries to peripheral or central nervous structures that are normally involved in signalling pain often result in painful symptoms, such as neuropathic pain (NP). The most typical traits of NP described by patients are paraesthesia, burning pain, shooting, electric-shock-like pain and evoked pain (hyperalgesia, allodynia). Paraesthesias (ant crawling = formication and tingling) are symptoms typically described by patients that are bothersome but not painful. There is almost always an area of abnormal sensation and the patient's maximum pain is often co-extensive with the area of sensory deficit. This is an important diagnostic feature for neuropathic pain. The sensory deficit is usually to noxious and thermal stimuli, which indicates damage to small-diameter afferent fibres or to the spinothalamic tract.21
In most cases within this study, the neuropathic area affected 60% of the extra-oral dermatome and 50% of the intra-oral dermatome supplied by the IAN. A previous report describes 85 patients in Korea presenting with post implant treatment nerve injury with neuropathic pain,22 which also appears to be a similar emerging issue to that of post endodontic pain.10,23 The general demographics of this patient cohort were similar to previous reports on implant surgery (Table 1).7,9,10,11,12,13,14,15,16
A significant deficiency in consent practice for implants has been previously reported.24 In this study, symptoms appeared to be aggravated by the lack of informed consent, which was given to only 30% of the patients, most of whom were not specifically warned about potential nerve injury. Over 70% of patients were referred six months post injury, which we now understand to be entirely inappropriate.
Subjective function and mechanosensory results of this study indicated and confirmed that symptoms experienced by patients with iatrogenic trigeminal nerve lesions can range from minimal anaesthesia in a small area, to devastating effects on the patient's quality of life.4 This is highly problematic, since patients anticipate improvements in their jaw function, dental, facial and even overall body image.25 Various assessment methods are required to quantify the level of discomfort experienced by patients, the associated functional disability and to diagnose the sensory impairment. Assessment should also provide accurate monitoring of sensory and functional recovery ideally with criteria for intervention where necessary.
NP is estimated to affect approximately 35% of chronic pain patients and up to 5% of the population.21,26 It has become apparent over the last five years that many patients undergoing surgery experience chronic NP as a result. Studies have reported the incidence of NP to be as high as 30-60% related to thoracotomy, breast surgery and herniorrhaphy surgery.26
Pre-operative planning and consent
Harris et al.27 recommend the use of cross sectional imaging techniques in the resorbed posterior mandible, for pre-operative radiographic evaluation before placement of implants. The introduction of cone beam computed tomography, which provides low radiation dose and high resolution 3D imaging, facilitates implant planning in critical sites. Planning software allows the path of the IAN to be drawn-in by an operator who may not necessarily be a clinician; however, the correct relationship must be confirmed by the individual actually carrying out the surgery.
Abarca et al.4 highlighted the necessity for cross-sectional imaging even for surgical procedures in the symphyseal region. Implants placed in proximity to the mental nerve region represented the highest risk, perhaps partly as a consequence of poor interpretation of radiographic views of the mental foramen and anterior loop of the IAN.14 The results reported in this paper highlight the need for an adequate safety zone in such cases. Selection of unnecessarily long implants may have further contributed to injury. Many authors suggest that 7 mm should be sufficient in routine cases28 and within this cohort many implants were longer than 10 mm. Use of shorter implants is recommended as a means to mitigate risk and increase the safety zone, with the specific aim to place implants with a safety zone of 2-4 mm.29,30 Clearly the extent of the required safety zone will be a function of the operators' surgical experience, as well as experience in radiographic interpretation. Clinicians must have an awareness that certain prep drills are up to 1.5 mm longer than the placed implant.
Intra-operative complications and recommendations
Intra-operative haemorrhagic complications were addressed in a recent article with an excellent review of local vascular anatomy.31 A sudden 'give' during preparation may be indicative of protrusion through the lingual or buccal plate but may also be associated with fracturing of the IAN canal roof, direct trauma, or extrusion of preparation debris into the canal. Another recent study evaluated the density and thickness of the bone that surrounds the mandibular canal and concluded that it was not able to resist the implant drill, and avoidance of excessive force was recommended.32
Previously reported intra-operative recommendations to prevent IAN injury in relation to implant bed preparation close to the IAN canal, include:
-
A recommendation to minimise the use of repeated IAN blocks33
-
Provide light operative LA and ask patient to indicate when they feel pain during the procedure (Articaine infiltrations and no IAN blocks)34
-
To use implant drills that have lost their sharpness35
-
To use drill 'stops'35 (The authors feel that this is an inappropriate recommendation as additional force would be required with a blunt drill)
-
Take intra-operative radiographs during implant bed preparation.36
If known section to the IAN has occurred then immediate referral to a specialist is recommended.
If an arterial or venous bleed is apparent, it may be advisable to not place the implant and wait two to three days before placing the implant in granulation tissue. The rationale is that if there is continued or excessive bleeding from the implant bed proximal to the ID canal (with likely perforation of the vessels there in), immediate placement of the implant may lead to 'strangulation' and secondary ischaemia of the IAN. Branemark is said to have recommended this verbally but we are unable to find this recommendation in previous publications.
Postoperatively
In this study, the information provided by the referring practitioner lacked detail. Three patients who were referred by e-mail or phone had their implants removed within 30 hours. Their injuries resolved fully suggesting that early removal may facilitate nerve injury resolution. This is indeed also recommended in a previous study17 and supported by the fact that several patients in this study had their implants removed between 30 hours and 6 days and none made a recovery. This reinforces the recommended practice of undertaking 'Homecheck' with in 12 hours post surgery to enable the patient to report ongoing neuropathy, which may dictate early removal of the implant is required. In addition severe or extreme pain post surgically may be an indicator of nerve damage and must not be overlooked by the treating clinician.
Adjunctive care and management procedures for this patient group are outlined in Table 3. A detailed consultation with a full explanation of their symptoms and nerve injury seemed to alleviate many concerns and anxieties, as seen by the 13 patients in this study who were discharged after 2-4 consultations. Early referral to a nerve specialist, as also suggested by Misch and Randolph,37 is therefore helpful for the management of patients with nerve injury. Honesty in estimating the likeliness of their injury improving also provides the patients with realistic goals. Only 15% of patients went on to have their implants restored, reflecting perhaps a loss of confidence in their treating dentist due to the injury.
Cognitive behavioural therapy (CBT) was of benefit to eight patients diagnosed with depression and significant depression with suicidal thoughts subsequent to their surgery. This recent development for management of chronic pain patients in our department is proving an invaluable and welcomed tool. In contrast to a study that reports the rather successful treatment of 85 patients with neuropathic pain using a 12-week course of pregabalin,22 we found that most patients could not cope with the side effects which included drowsiness, loss of memory and remoteness; preferring to tolerate the pain.
A recent report suggests, somewhat optimistically, that nerve injuries related to implants can be corrected using microsurgical repair and advocate early intervention.7 The authors believe that if a nerve injury is suspected, a basic neurosensory examination is required, (neuropathic area and pain experience, altered sensation or numbness), ideally within the day of surgery. A simple phone call four to six hours post surgery will enable the surgeon to ascertain if neuropathy is present. If IANI is evident then the implant should be removed within 24 hours of placement. Removal after osseointegration is unlikely to resolve the nerve injury.38 In these cases, the dentist no longer removes the implant, as it appears to be of little value in reversing nerve damage and associated symptoms.
Medico-legal aspect
Increasingly, patient complaints received by the UK General Dental Council (GDC) and the American Dental Association (ADA) are implant related. Of claims made against the American Dental Insurance companies (Fortress and OMSNIC) 24% of claims are related to nerve injury. According to a Riskwise Medical Protection Society publication in 2010,39 6% of 9,000 MPS cases in 2007 were implant related, rising from 4% in 2003. Twenty-four percent of oral surgery dental implant claims have an average payment of $89,000 per patient, while 37% of the general dental implant claims had an average payment of $63,000.40 In 2007, total settlement sums of £20-30K were common in the UK with some payments reaching £50-100K. 25% of all cases exceeding £150K were implant related. Implant-related nerve injuries average payouts are higher than the average payout for IAN injury related to third molar surgery in the US.
The most significant issue with implant related nerve injuries are that they are avoidable, and potentially permanent with or without surgical intervention.41 There are several reports emerging of delayed onset pain with implants. Other reports of persistent post surgical pain post implant placement are similar to reports on post endodontic pain.42,43,44 It is likely that this post surgical neuropathic pain may be associated with nerve injury and should be prevented and treated accordingly.
In conclusion, iatrogenic implant related nerve injury often causes persistent neuropathic pain with significant associated functional problems which seriously affects quality of life. Evidence of proper consent and pre-operative radiographic planning was sparse. Clinicians therefore need to ensure that all patients are adequately consented and that pre-operative radiographic planning is carried out. Postoperative follow up, which was often inadequate, needs to improve. Prompt removal of implants needs to be carried out to minimise chances of developing chronic irreversible post surgical neuropathy.
References
Caissie R, Goulet J, Fortin M, Morielli D . Iatrogenic paresthesia in the third division of the trigeminal nerve: 12 years of clinical experience. J Can Dent Assoc 2005; 71: 185–190.
Renton T, Yilmaz Z . Profiling of patients presenting with posttraumatic neuropathy of the trigeminal nerve. J Orofac Pain 2011; 25: 333–344.
Ziccardi V B, Assael L A . Mechanisms of trigeminal nerve injuries. Atlas Oral Maxillofac Surg Clin North Am 2001; 9: 1–11.
Abarca M, van Steenberghe D, Malevez C, De Ridder J, Jacobs R . Neurosensory disturbances after immediate loading of implants in the anterior mandible: an initial questionnaire approach followed by a psychophysical assessment. Clin Oral Investig 2006; 10: 269–277.
Elian N, Mitsias M, Eskow R et al. Unexpected return of sensation following 4.5 years of paresthesia: case report. Implant Dent 2005; 14: 364–367.
Robinson P P, Smith K G . How clinical research changed the habit of a lifetime. Br Dent J 1997; 183: 238.
Hegedus F, Diecidue R J . Trigeminal nerve injuries after mandibular implant placement – practical knowledge for clinicians. Int J Oral Maxillofac Implants 2006; 21: 111–116.
Ziccardi V B, Zuniga J R . Nerve injuries after third molar removal. Oral Maxillofac Surg Clin North Am 2007; 19: 105–115, vii.
Balshi T J . Preventing and resolving complications with osseointegrated implants. Dent Clin North Am 1989; 33: 821–868. Review.
Delcanho R E . Neuropathic implications of prosthodontic treatment. J Prosthet Dent 1995; 73: 146–152.
Rubenstein J E, Taylor T D . Apical nerve transection resulting from implant placement: a 10-year follow-up report. J Prosthet Dent 1997; 78: 537–541.
Wismeijer D, van Wass M A, Vermeeren J I, Kalk W . Patient's perception of sensory disturbances of the mental nerve before and after implant surgery: a prospective study of 110 patients. Br J Oral Maxillofac Surg 1997; 35: 254–259.
Bartling R, Freeman K, Kraut R A . The incidence of altered sensation of the mental nerve after mandibular implant placement. J Oral Maxillofac Surg 1999; 57: 1,408–1,412.
Walton J N . Altered sensation associated with implants in the anterior mandible: a prospective study. J Prosthet Dent 2000; 83: 443–449.
von Arx T, Häfliger J, Chappuis V . Neurosensory disturbances following bone harvesting in the symphysis: a prospective clinical study. Clin Oral Implants Res 2005; 16: 432–439.
Hillerup S . Iatrogenic injury to oral branches of the trigeminal nerve: records of 449 cases. Clin Oral Investig 2007; 11: 133–142.
Khawaja N, Renton T . Case studies on implant removal influencing the resolution of inferior alveolar nerve injury. Br Dent J 2009; 206: 365–370.
Robinson P P, Smith K G, Johnson F P, Coppins D A . Equipment and methods for simple sensory testing. Br J Oral Maxillofac Surg 1992; 30: 387–389.
Renton T, Hankins M, Sproate C, McGurk M . A randomised controlled clinical trial to compare the incidence of injury to the inferior alveolar nerve as a result of coronectomy and removal of mandibular third molars. Br J Oral Maxillofac Surg 2005; 43: 7–12.
Renton, T, Thexton A, Crean S J, Hankins M . Simplifying the assessment of the recovery from surgical injury to the lingual nerve. Br Dent J 2006; 200: 569–573.
Rehm S, Koroschetz J, Baron R . An update on neuropathic pain. Eur Neur Rev 2008; 3: 125–127.
Park J H, Lee S H, Kim S T . Pharmacologic management of trigeminal nerve injury pain after dental implant surgery. Int J Prosthodont 2010; 23: 342–346.
Gregg J M . Neuropathic complications of mandibular implant surgery: review and case presentations. Ann R Australas Coll Dent Surg 2000; 15: 176–180.
Kotrashetti V S, Kale A D, Hebbal M, Hallikeremath S R . Informed consent: a survey of general dental practitioners in Belgaum city. Indian J Med Ethics 2010; 7: 90–94.
Kiyak H A, Beach B H, Worthington P, Taylor T, Bolender C, Evans J . Psychological impact of osseointegrated dental implants. Int J Oral Maxillofac Implants 1990; 5: 61–69.
Kehlet H, Jensen T S, Woolf C J . Persistent postsurgical pain: risk factors and prevention. Lancet 2006; 367: 1,618–1,625.
Harris D, Buser D, Dula K et al. E. A. O. guidelines for the use of diagnostic imaging in implant dentistry. A consensus workshop organized by the European Association for Osseointegration in Trinity College Dublin. Clin Oral Implants Res 2002; 13: 566–570.
Maló P, de Araújo Nobre M, Rangert B . Short implants placed one-stage in maxillae and mandibles: a retrospective clinical study with 1 to 9 years of follow-up. Clin Implant Dent Relat Res 2007; 9: 15–21.
Greenstein G, Tarnow D The mental foramen and nerve: clinical and anatomical factors related to dental implant placement: a literature review. J Periodontol 2006; 77: 1,933–1,943.
Misch C E, Crawford E A . Predictable mandibular nerve location – a clinical zone of safety. Int J Oral Implantol 1990; 7: 37–40.
Lamas Pelayo J, Peñarrocha Diago M, Martí Bowen E . Intraoperative complications during oral implantology. Med Oral Patol Oral Cir Bucal 2008; 13: E239–243.
Başa O, Dilek O C . Assessment of the risk of perforation of the mandibular canal by implant drill using density and thickness parameters. Gerondontology 2011; 28: 213–220.
Renton T, Adey-Viscuso D, Meechan J G, Yilmaz Z . Trigeminal nerve injuries in relation to the local anaesthesia in mandibular injections. Br Dent J 2010, 209: E15.
Smith D W, Peterson M R, DeBerard S C . Local anaesthesia. Topical application, local infiltration and field block. Postgrad Med 1999; 106: 57–60, 64–66.
Sharawy M, Misch C E, Weller N, Tehemar S . Heat generation during implant drilling: the significance of motor speed. J Oral Maxillofac Surg 2002; 60: 1,160–1,169.
Burstein J, Mastin C, Le B . Avoiding injury to the inferior alveolar nerve by routine use of intraoperative radiographs during implant placement. J Oral Implantol 2008; 34: 34–38.
Misch C E, Resnik R . Mandibular nerve neurosensory impairment after dental implant surgery: management and protocol. Implant Dent 2010; 19: 378–386.
Bert M . Complicaciones y fracasos en implantes osteointegrados. Causas, tratamiento, prevención. Barcelona: Masson; 1995.
Renton T, Yilmaz Z . Iatrogenic injuries to oral trigeminal nerve branches: 221 cases. Risk management from dental protection. Riskwise UK 2009; 39: 4–9.
Renton T . Prevention of iatrogenic inferior alveolar nerve injuries in relation to dental procedures. Dent Update 2010; 37: 350–352, 354–356, 358–360.
Pogrel M A, Thamby S . Permanent nerve involvement resulting from inferior alveolar nerve blocks. J Am Dent Assoc 2000; 131: 901–907.
Rodríguez-Lozano F J, Sanchez-Pérez A, Moya-Villaescusa M J, Rodríguez-Lozano A, Sáez-Yuguero M R . Neuropathic orofacial pain after dental implant placement: review of the literature and case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010; 109: e8–e12.
Clark G T . Persistent orodental pain, atypical odontalgia and phantom tooth pain: when are they neuropathic disorders? J Calif Dent Assoc 2006; 34: 599–609.
Queral-Godoy E, Vazquez-Delgado E, Okeson J P, Gay-Escoda C. Persistent idiopathic facial pain following dental implant placement: a case report. Int J Oral Maxillofac Implants 2006; 21: 136–140.
Author information
Authors and Affiliations
Additional information
Refereed paper
Rights and permissions
About this article
Cite this article
Renton, T., Dawood, A., Shah, A. et al. Post-implant neuropathy of the trigeminal nerve. A case series.. Br Dent J 212, E17 (2012). https://doi.org/10.1038/sj.bdj.2012.497
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bdj.2012.497
This article is cited by
-
Comparative analysis of mandibular anatomical variations between panoramic radiography and cone beam computed tomography
Oral and Maxillofacial Surgery (2014)
-
Summary of: Post-implant neuropathy of the trigeminal nerve. A case series.
British Dental Journal (2012)