Key Points
-
Identifies distinct indications for the post-operative use of flumazenil in specifically selected cases.
-
Demonstrates the safe and appropriate use of midazolam and flumazenil for conscious sedation.
-
Makes recommendations about the administration of flumazenil and discharge of patients who have undergone midazolam-induced conscious sedation for dental procedures.
Abstract
Objective To investigate the use of flumazenil after midazolam-induced conscious sedation.
Design and setting A prospective audit was carried out in the Department of Sedation and Special Care Dentistry at Guy's Hospital, King's College, London, 2009.
Subjects Patients sedated with midazolam for dental treatment.
Method All clinical staff completed the data capture proforma when flumazenil was administered to a patient after sedation with midazolam.
Results Four hundred and fifty-three patients were sedated with midazolam. Flumazenil was used in 32 cases. No cases required flumazenil for the emergency treatment of respiratory depression.
Conclusions The results of the audit confirmed the safe and appropriate use of midazolam for conscious sedation within the Department of Sedation and Special Care Dentistry at Guy's Hospital and demonstrated that flumazenil use was low and in accordance with current best practice. The audit has highlighted distinct indications for the post-operative use of flumazenil in specifically selected cases. Each case should be individually considered, justified and documented within the patient's clinical record.
Similar content being viewed by others
Background
The National Patient Safety Agency (NPSA)'s rapid response report (RRR), Reducing risk of overdose with midazolam injection in adults,1 reviewed 1,529 medication-related patient safety incident reports received by the Reporting and Learning System (RLS) where the words midazolam or flumazenil (or related terms) were contained in the report. The report documented 498 incidents of adult patients being given midazolam during the period from November 2004 to November 2008. Of the 498 incidents, three patients died, 48 incidents resulted in moderate harm to patient and the remaining 447 caused low or no harm to the patients. Of the 1,529 reports only two were attributed to dentistry. One of the dental-related incidents was documented in the report:
'Incident 8 - Incomplete reversal
During IVS procedure pt re-sedated. Flumazenil given at 1420 – flumazenil has a shorter half-life than midazolam. Pt woke for about 40 minutes then re-sedated. SATS initially normal (brought back from waiting room to surgery). Dentist went for advice from Day Surgery Unit while Dental Nurse managed SPO2 drop by managing airway. Pt recovered consciousness shortly afterwards.' 1
The report concluded that the NPSA had identified serious problems in the current use of midazolam for conscious sedation in adults. Errors were reported to have occurred because of lack of training in the use of midazolam, problems in titration and dosing (including use of part-ampoules and vials of higher strength) for individual patients. It also reported that the use of flumazenil as an antidote to midazolam overdose is widespread and that, although useful to reverse the effects of over-sedation, it is not without risk.1
In addition, and of particular concern, was evidence elicited from the 2004 National Confidential Enquiry into Patient Outcome and Death (NCEPOD).2 This report reviewed 1,818 inpatient deaths within 30 days of interventional gastrointestinal endoscopy in hospitals in England, Wales and Northern Ireland. The NCEPOD advisors judged that the sedation given was inappropriate in 14% of these cases, usually because an overdose of benzodiazepine had been administered. Detailed analysis of the fatalities data showed that patients who had received flumazenil died two days sooner than those who had not received it.2
The purpose of the RRR was to alert healthcare staff involved in prescribing, administration or supply of injectable midazolam for use in adult conscious sedation to the risk of overdose or harm. It outlined clear actions, which apply to all organisations in the NHS and independent sector, to minimise risks of overdosing and subsequent harm from midazolam in adults.
Objectives
-
To identify the reasons for use of flumazenil in the Department of Sedation and Special Care Dentistry at Guy's Hospital
-
To identify how flumazenil is administered to patients
-
To formulate recommendations to ensure the appropriate use of flumazenil following midazolam-induced conscious sedation.
Method
A prospective audit was carried out to identify the use of flumazenil in the Department of Sedation and Special Care Dentistry at Guy's Hospital.
All clinical staff, including postgraduate diploma and MSc students, were asked to complete a proforma when flumazenil was used following intravenous sedation with midazolam. Data collection commenced on 1st April 2009 for 12 weeks.
Data was captured using a simple proforma (Fig. 1), designed to be quick and easy to complete. Rather than a 'tick-box' structure the proforma allowed the clinician to give a reason for the administration of flumazenil and any further information that they deemed necessary regarding the administration.
Results
The total number of patients given midazolam for dental treatment over the audit period in the Department of Sedation and Special Care Dentistry was 453.
A total of 32 proformas were completed detailing the use of flumazenil following midazolam-induced conscious sedation during this period, this was equivalent to 7% of the sedation treatment episodes.
All of the completed proformas provided the required information regarding the time of midazolam administration, details of why flumazenil was administered, details of amount and method of flumazenil administration and time of administration; all but one of the proformas gave the time of discharge from the department.
Reasons for flumazenil use
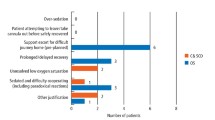
The justifications given for the use of flumazenil following midazolam-induced conscious sedation are shown in Figure 2. These results are demonstrated in percentage form in Figure 3. These results reveal that flumazenil was used to augment recovery for cases exhibiting prolonged recovery times and for complicating problems like post-operative retching/nausea which had not been highlighted in previous literature.
Method of administration of flumazenil
Results detailing the method of administration of flumazenil are shown in Figure 4. All patients administered flumazenil were given a dose of 0.5 mg. Of the 32 patients given flumazenil, 31 were administered flumazenil via the intravenous route. Of these, the majority (21) were administered flumazenil in increments and the remainder (nine) by bolus injection.
One patient was administered flumazenil intranasally. This patient had Rett syndrome, a neuro-developmental condition, characterized by normal early development followed by loss of purposeful use of the hands, distinctive hand movements, slowed brain and head growth, gait abnormalities, seizures, and mental disability. Intravenous access had been established but was no longer patent when administration of flumazenil was necessary; consequently flumazenil was given intranasally in two divided smaller bolus doses.
Prolonged recovery
Prolonged recovery was given as a reason for using flumazenil after midazolam-induced conscious sedation for 22 patients.
In addition to prolonged recovery, extra information to support flumazenil use was provided for 16 patients. Figure 5 shows the additional information provided. In some cases, more than one additional reason was provided to support flumazenil use, hence the total number of reasons for giving flumazenil (22) is greater than the number of additional information reports (16).
Flumazenil administration
The period of time between midazolam administration and flumazenil administration is shown in Figure 6. 'Minutes after midazolam' relates to the time (minutes) that flumazenil was administered after the induction of sedation with midazolam. The graph shows two peaks at 35 minutes and 60 minutes. These peaks are attributed to administration of flumazenil to patients for reasons other than prolonged recovery (35 minutes) and for prolonged recovery (60 minutes) respectively.
Comparison of the groups
An unpaired t-test was used to assess the difference between the two groups. A 95% confidence interval demonstrated that there was no statistical difference between the groups (t = 1.48).
Discharge time
Following administration of flumazenil, patients were discharged from the department into the care of their escorts as demonstrated in Figure 7. The results demonstrated that patients given flumazenil for prolonged recovery were deemed to have recovered sufficiently to be discharged to the care of their escorts between 5 and 30 minutes after administration of flumazenil. One proforma did not provide the discharge time and, therefore, was not included in the statistical analysis.
For prolonged recovery cases the mean time to discharge was 17.75 minutes after flumazenil administration; the mode was 10 minutes. For cases other than prolonged recovery, the mean was 17.5 minutes and the mode was indeterminate between 10 and 15 minutes.
The data showed that patients who were given flumazenil for reasons other than prolonged recovery were deemed to have recovered sufficiently to be discharged within 10 and 30 minutes following administration of flumazenil.
Comparison of the groups
An unpaired t-test with a 95% confidence interval demonstrated that there was no statistical difference between the two groups (t = 0.18).
Conclusion
The use of flumazenil after midazolam-induced conscious sedation within the Department of Sedation and Special Care Dentistry at Guy's Hospital was low (7%). No patients required emergency administration of flumazenil for treatment of overdose or respiratory depression. The audit has highlighted distinct indications for the post-operative use of flumazenil in specifically selected cases. Each case should be individually considered, justified and documented within in the patient's clinical record.
Discussion and recommendations
Flumazenil use
A recent study by Ransford et al. documented 316 episodes of sedation with midazolam administered to adults with severe disabilities, it reported use of flumazenil to reverse sedation in approximately 1 in 5 (20%) episodes, often to manage patients attempting to get up before properly recovered.3 No other studies were identified for comparison, consequently the use of flumazenil after midazolam-induced conscious sedation within the Department of Sedation and Special Care Dentistry at Guy's Hospital, King's College Dental Institute during this study was concluded to be low (7%).
Indications for flumazenil use
Flumazenil injection is licensed in the UK for the complete or partial reversal of the central sedative effects of benzodiazepines. It is used in outpatient departments, in anaesthesia and intensive care for:
-
The termination of general anaesthesia induced and/or maintained with benzodiazepines
-
The reversal of benzodiazepine sedation in short diagnostic and therapeutic procedures
-
The specific reversal of the central effects of benzodiazepines (respiratory depression or arrest), to allow return to spontaneous respiration and consciousness, in patients in intensive care.1
Other indications for flumazenil use in conscious sedation are, however, more ambiguous. The following have been described in the literature as indications for reversal of conscious sedation for dentistry:
-
Over-sedation
-
Difficult journey home
-
Patients with learning difficulties
-
Escort/patient difficulties.4
Patients with special needs or learning difficulties may display fractious, unpredictable behaviour that could be difficult for the carer to manage; for these patients the use of flumazenil would be appropriate.
Escort/patient difficulties may become evident if there is a disparity between the sizes of the escort and the patient; a small escort may struggle to manage a large patient whom is still experiencing some effects of the sedation. In this circumstance the use of flumazenil to assist the escort would be appropriate.
Recommendation
This audit has identified the safe use of flumazenil in a number of carefully selected cases that had previously not been highlighted in the literature.
It is recommended that when flumazenil is administered for reasons other than an emergency, the decision should be made for each individual case, justified and documented within the clinical record.
Normal recovery
Recovery from anaesthesia is defined as 'The period from the end of surgery to when the patient is alert and physiologically stable'.5 Defining normal recovery is difficult because drowsiness may persist for many hours.
Defining recovery from sedation is also complicated and no definition was identified in the literature. Following conscious sedation with midazolam, normal recovery may differ from recovery following general anaesthesia in that physiological stability does not relate to physical competence. Recovery from sedation could be defined as 'the period from the end of treatment to a point when the patient is physiologically stable, alert, orientated in time and space and able to walk unaided'. This adds a level of physical competence to the physiological parameters in the definition of recovery from anaesthesia.
In consideration of the elimination half-life (90–180 minutes) and distribution half-life (6-20 minutes) of midazolam some signs of recovery could be expected between 30 and 60 minutes after administration of the last increment of sedative agent.
Normal recovery could also be affected by confounding factors in the patient's medical history, the method and speed of administration of the sedative agent, the total dose and the route of administration.
Prolonged recovery
What clinicians considered 'prolonged recovery' varied. Figure 6 shows the range of times (minutes after midazolam administration) after which flumazenil was administered to patients who were deemed to be experiencing prolonged recovery. In this study, the clinicians deemed patients to require flumazenil administration for prolonged recovery when recovery was in excess of 25 minutes after induction of sedation with midazolam.
Literature suggests a sedative period gives 25 minutes of profound sedation followed by 20 minutes of light sedation,4 hence those patients given flumazenil for prolonged recovery within 45 minutes after midazolam administration were likely still to be within the sedation phase rather than the recovery phase.
Following midazolam administration the duration of dental treatment can vary considerably. For example, some routine restorative treatments can last up to 60 minutes, yet a simple extraction of a tooth may last less than 15 minutes. Hence, at completion of treatment, patients will be at different points in the recovery process.
In addition to managing phobia, midazolam can be used to obtund severe gag reflexes to enable dental treatment to be carried out. In these cases the level of sedation may be minimal and the doses used may vary. Consequently the dose and reason for midazolam use must be considered when assessing prolonged recovery. The point at which flumazenil is given will relate to the depth of sedation necessary to carry out the treatment and the length of time taken to complete the procedure.
The importance of the clinician's assessment of the patient's cognitive and physical capabilities before case-selection and decision to administer flumazenil to enhance recovery before discharge cannot be over-emphasised.
Definition of early discharge following midazolam sedation
The following statement is a proposed definition of early discharge: 'Early discharge is when a patient is released from care before the patient has achieved adequate physiological stability, mental awareness and orientation and physical ability to walk unaided and before the clinician assesses that the patient has entered an appropriate stage of recovery.'
Clinicians should not discharge patients during this time (but some patients may self-discharge against clinician advice). With very short dental treatments the patient may still be within the active sedation phase for some time after all dental treatment has ceased. Administration of flumazenil and discharge during this time/phase may be considered 'early' and is inappropriate.
Definition of prolonged recovery
The following statement is a proposed definition of prolonged recovery. 'Prolonged recovery can be assessed as occurring when a physiologically stable patient has regained sufficient mental alertness to be discharged but is not yet demonstrating adequate physical signs of normal recovery more than 45 minutes after administration of the last increment of midazolam.'
Recommendation
It is suggested that administration of flumazenil may be indicated in case-selected patients experiencing prolonged recovery and particularly where there are confounding factors such as medical, mental, learning, physical disability or social complications.
Method of administration of flumazenil
Within this study, 31 patients were given 0.5 mg flumazenil administered intravenously; the majority of these were administered incrementally, a few were given the drug as a slow bolus injection. One patient was administered flumazenil intranasally.
Incremental versus bolus administration
All of the patients were administered a total dose of 0.5 mg of flumazenil. Administration of small amounts of the drug, combined with observation of the effects between each administration, up to the total dose was considered to be incremental administration. More rapid administration of the total dose of the drug was considered to be bolus administration.
Intranasal flumazenil
Flumazenil is currently licensed for intravenous administration. In the case of one patient for whom flumazenil could not be administered intravenously flumazenil was administered in 2 bolus doses intranasally using an atomization device.
Intranasal drug delivery is supported by recent research into new forms of drug delivery and is emerging as a promising method of delivering medications directly to the bloodstream through absorption across the nasal mucous membranes.
A study by Scheepers et al.6 indicated that plasma levels attained after intranasal administration of flumazenil were similar to those reported after intravenous administration, and may be sufficient to antagonise the side effects of benzodiazepines.
Advantages of intranasal administration of drugs
The rich vascular plexus of the nasal cavity provides a direct route into the bloodstream for medications that easily cross mucous membranes. Due to direct absorption into the bloodstream, rate and extent of absorption and plasma concentration is comparable to that obtained by the intravenous route. Administration is essentially painless, and is immediately and readily available to most patients.
Disadvantages of intranasal administration of drugs
A percentage of the drug, administered intrasally, may pass to the oropharynx and be ingested. Absorption through the stomach will become subject to first pass metabolism by the liver and reduce bioavailability. Absorption time is also increased.
Recommendation
For emergency reversal of acute respiratory depression during conscious sedation flumazenil should be administered as a slow bolus dose of not less than 0.5 mg (repeated once if necessary).
For elective use in case selected individuals, flumazenil should be administered incrementally. It is recommended that 0.2 mg should be administered initially over 15 seconds. After 45 seconds further increments of 0.1-0.2 mg can be given and repeated at 60-second intervals where necessary up to a maximal total dose of 1 mg.
Discharge of patients following flumazenil administration
The literature has documented that flumazenil does not fully reverse the effects of midazolam on psychomotor or cognitive ability.7,8,9,10,11 Initial onset of action is very rapid, often within one minute of administration. As recommended by the Roche data sheet12 care should be taken to assess the patient for any complications for an adequate period of time following flumazenil administration. No definitive time is stated.
The importance of providing post-operative instructions and aftercare advice to both the escort and patient has been reported and is widely accepted.7,8,12,13,14
Once again, despite the administration of flumazenil, emphasis is given to the importance of the clinician's assessment of the patient's cognitive and physical capabilities before discharge; the potential for residual sedation and the effects of flumazenil in ambulatory patients should be considered.
Recommendation
An adequate period of time to assess and monitor patients before discharge appears to be between 10 and 15 minutes following flumazenil administration. Patients should not be discharged before they have demonstrated adequate physiological, mental and physical signs of normal recovery from sedation.
Summary
An audit project to investigate the use of flumazenil within the Department of Sedation and Special Care Dentistry at Guy's Hospital, King's College Dental Institute, was completed in accordance with the recommendations made by the NPSA's rapid response report Reducing risk of overdose with midazolam injection in adults.
The results have demonstrated that the use of flumazenil within the department was low (7%). During the period of the audit, no patients required administration of flumazenil for emergency reversal of overdose or respiratory depression.
The audit has highlighted distinct indications for the post-operative use of flumazenil in specifically selected cases. Each case should be individually considered, justified and documented within in the patient's clinical record.
References
National Patient Safety Agency. Rapid response report: reducing risk of overdose with midazolam injection in adults. London: National Patient Safety Agency, 2008.
National Confidential Enquiry into Patient Outcome and Death. Scoping our practice. The 2004 report of the National Confidential Enquiry into Patient Outcome and Death. London: National Confidential Enquiry into Patient Outcome and Death, 2004.
Ransford N J, Manley M C, Lewis D A et al. Intranasal/intravenous sedation for the dental care of adults with severe disabilities: a multicentre prospective audit. Br Dent J 2010; 208: 565–569.
Meechan J G, Robb N D, Seymour R A (eds). Pain and anxiety control for the conscious dental patient. 1st ed. Oxford: Oxford University Press, 1998.
Yentis S M, Hirsch N P, Smith G B (eds). Anaesthesia and intensive care. An encyclopaedia of principles and practice. 3rd ed. Edinburgh: Butterworth Heinemann, 2004.
Sheepers L D, Montgomery C J, Kinahan A M et al. Plasma concentration of flumazenil following intranasal administration in children. Can J Anaest 47: 120–124, Feb 2000.
Girdler N M, Fairbrother K J, Lyne J P et al. A randomised crossover trial of post-operative cognitive and psychomotor recovery from benzodiazepine sedation: effects of reversal with flumazenil over a prolonged recovery period. Br Dent J 2002; 129: 335–339.
Coulthard P, Sano K, Thomson P J, Macfarlane T V . The effects of midazolam and flumazenil on psychomotor function and alertness in human volunteers. Br Dent J 2000; 188: 323–328.
Curran H C, Birch B . Differentiating the sedative, psychomotor and amnesic effects of benzodiazepines: a study with midazolam and the benzodiazepine antagonist flumazenil. Psychopharmacology (Berl) 1991; 103: 519–523.
Davies C A, Sealey C M, Lawson J L, Grant I S . Reversal of midazolam sedation with flumazenil following conservative dentistry. J Dent 1990; 18: 113–118.
Hunter K M, Zacharias M, Parkinson R, Luyk N H . Effect of flumazenil on the recovery from intravenous midazolam. N Z Dent J 1994; 90: 912.
Roche Pharmaceuticals. Flumazenil (Romazicon) data sheet. Revised April 2010. Available at http://www.gene.com/gene/products/information/romazicon/pdf/pi.pdf.
Naeve N, Reid C, Scholey A B et al. Dose-dependent effects of flumazenil on cognition, mood and cardio-respiratory physiology in healthy volunteers. Br Dent J 2000; 189: 668–674.
Rodrigo C . Flumazenil in dentistry. Anesth Prog 1995; 42: 121–125.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Henthorn, K., Dickinson, C. The use of flumazenil after midazolam-induced conscious sedation. Br Dent J 209, E18 (2010). https://doi.org/10.1038/sj.bdj.2010.1132
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bdj.2010.1132
This article is cited by
-
Re-audit of the use of flumazenil following midazolam-induced conscious sedation
BDJ Open (2023)
-
Audit of flumazenil use in special care and oral surgery sedation services
British Dental Journal (2021)
-
Midazolam use for dental conscious sedation: how safe are we?
British Dental Journal (2018)
-
Safety and Efficacy of Flumazenil for Reversal of Iatrogenic Benzodiazepine-Associated Delirium Toxicity During Treatment of Alcohol Withdrawal, a Retrospective Review at One Center
Journal of Medical Toxicology (2014)
-
Summary of: The use of flumazenil after midazolam-induced conscious sedation
British Dental Journal (2010)