Abstract
Introduction:
Spinal cord injuries in new born infants following a traumatic delivery or umbilical cord catheterisation due to thromboembolism are well known. Cases with atraumatic acute onset of neonatal paraplegia have also been described in preterm babies or babies born small for gestational age with a stormy postnatal course related to ischaemic aetiology. We describe a rare case of infarction of the spinal cord from a predominant haemorrhagic aetiology.
Case presentation:
A term female baby, first child of unrelated parents, was born by normal vaginal delivery. She had meconium aspiration at birth, leading to severe respiratory distress, requiring neonatal intensive care admission. At 2 weeks, she developed new flaccid paraplegia. MRI scan of the spine showed haemorrhagic infarction of the spinal cord from the level of thoracic inlet, vertebral level C7–T1. A follow-up MRI scan at 11 months revealed severe atrophy of the cord distal to C6. At 3 years of age, she had good upper-limb function, diaphragmatic breathing and flaccid paralysis of lower limbs.
Discussion:
In an acutely unwell term infant with symptoms of paralysis or spinal cord damage, haemorrhagic infarction needs to be considered in the differential diagnosis. To our knowledge, this is the first reported case of spinal cord injury in a term infant with a haemorrhagic lesion, and it helps to understand the pathogenesis of nontraumatic insult.
Similar content being viewed by others
Introduction
Spinal cord injuries (SCI) in new born infants following a traumatic delivery1 or umbilical cord catheterisation due to thromboembolism of artery are well known.2,3,4 Hypoxic–ischaemic SCI after perinatal asphyxia has been reported, although the clinical picture is primarily cerebral.5,6,7,8 Cases with atraumatic acute-onset neonatal paraplegia have also been described in preterm babies or babies born small for gestational age with a stormy postnatal course related to ischaemic aetiology.9 Arendar et al.10 in largest neonatal nontraumatic case series of 30 patients described SCI with sepsis, respiratory tract compromise, mechanical ventilation, polycythaemia, anaemia, umbilical catheterisation and exchange transfusion.10 All of these cases had ischaemic aetiologies. We describe a rare case of infarction of spinal cord with predominant haemorrhagic aetiology in a sick term neonate and the outcome of radiological and clinical features. Parents of this child consented for the publication.
Case presentation
A female child of unrelated parents and uncomplicated pregnancy was delivered at term plus 3 days by normal vaginal delivery. She had meconium aspiration at birth. She was immediately ventilated and transferred to intensive care. She was critically ill as oxygenation was suboptimal despite high-frequency oscillatory ventilation. She had developed persistent pulmonary hypertension. She had umbilical arterial and venous catheters. She was on full ionotropic support. Echocardiogram showed mild impairment of left ventricular function. Venous access was difficult, and on day 4 she had left femoral line insertion; but, in an attempt to do so, her right femoral artery was hit; hence, she required a 24 h heparin infusion. At day 5, her platelet count dropped to 85×109 l−1 (normal range: 150–450×109 l−1), and her C-reactive protein was high at 40 (normally <5), suggesting sepsis. She was commenced on intravenous antibiotics. On day 9, the prothrombin time was 14 s (normal range: 10–14 s), fibrinogen level was 632 mg dl−1 (normal range: 170–450 mg dl−1), activated partial thromboplastin time was <25 (25–38 s) and International Normalised ratio was 1.2. She had received vitamin K at birth. She continued to receive treatment for sepsis.
On day 11, she was extubated to high-flow nasal oxygen and was maintaining oxygen saturations between 91 and 97%. On day 12, cardiac scan showed thrombus in ductus venous, aortic arch turbulence and mild pulmonary stenosis. She was commenced on aspirin for thromboprophylaxis and briefly intubated for transfer to tertiary centre for an echocardiograph. Later, the transfer was abandoned as she was clinically stable and she was extubated to high-flow oxygen.
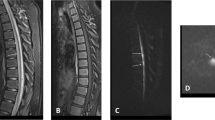
Forty-eight hours later, she was noted to have reduced movements of lower limbs. This had not been reported before at daily ward rounds or during nursing care. She was alert, had a soft cry, positive gag reflex, spontaneous diaphragmatic breathing, antigravity movements of the upper limbs, protruding hypotonic abdomen, hypotonic lower limbs and absence of abdominal and deep tendon reflexes in the lower limbs. MRI scan within 24 h confirmed infarction of spinal cord extending from C7 to T1 (Figure 1). The axial T2W MR image at T3 (thoracic) level shows a swollen cord with intramedullary high signal. On the T1W-weighted images, there is high signal in the spinal canal at multiple thoracic and lumbar levels, which could be due to haemorrhage (Figure 1). There was no evidence of soft tissue damage to suggest traction injury. The following weeks were troubled with episodes of autonomic instability with hypothermia and temperature reducing to 34 °C and bradycardia. She was discharged home at 3 months of age.
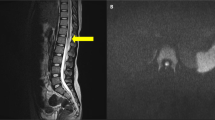
MRI features of the spinal cord. (1a) Sagittal T2W, (1b) T1W and (1c) Gadolinium enhanced T1W MR images 24 h post paralysis show a swollen cord at the cervicothoracic junction. The post contrast image (1c) also shows an abrupt junction between viable and infarcted cord at the C7 level (arrow). (1d) The axial T2W MR image at T3 (thoracic) level shows a swollen cord with intramedullary high signal. The appearances are consistent with infarction at the cervicothoracic junction. On the (1b) T1W weighted images there is high signal in the spinal canal at multiple thoracic and lumbar levels, which could be due to haemorrhage. (2a) Sagittal T1W, (2b, 2c) consecutive sagittal T2W MR images 11 months later show atrophic change and abrupt termination of cord (arrow) at the C6 level. Below this level, no cord tissue can be seen. Also note that there is low signal along the dura throughout the thoracic and lumbar spine suggestive of hemosiderin possibly a result of old haemorrhage.
Eleven months post SCI, she continued to have flaccid paralysis of legs with no sign of neurological recovery. Repeat MRI scan showed severe atrophy of the cord distal to C6 (Figure 1). MRI of the brain was normal. Motor abilities for rest of the body at 3 years of age had consistently improved with good head control, ability to sit up from the lying position, maintaining sitting with minimal support, reaching out for things with upper limbs and easily raising arms above the level of shoulder. The neurologic exam of the upper limbs is completely intact. She does not have appreciation of sensations from T6 downward. Further sensory and motor neurological examination is not possible due to her young age. She is showing improvement in other areas of development like speech and language. Both hips are uncovered approximately 50% using Reimers index. It is not affecting seating or causing discomfort. She has curvature of the spine with C-shaped scoliosis, with an apex at the thoracolumbar junction (Cobb angle 27 degrees) and exaggerated lumbar lordosis. There is asymmetry of the ribs. Bowel movements occur on alternate days with support of lactulose. Bladder management is by intermittent catheterisation. Renal ultrasound shows scarring of right lower renal pole. Her skin has remained healthy and intact.
Discussion
The vascular supply of spinal cord is provided by the anterior and two paired posterior spinal arteries (longitudinal direction). The main share of blood flow to the spinal cord is felt to come through the regional aortic branches of the anterior spinal artery.11 In addition to these three arteries, additional blood supply comes from branches of the vertebral arteries in the upper cervical region and variable branches of subclavian artery in the lower cervical spine.11,12 The anterior portion of the thoracic, lumbar and sacral supply is reinforced by segmental arteries from the aorta, intercostal arteries and internal iliac arteries. The anterior spinal artery is not necessarily continuous nor are the regional branches able to compensate for other vascular areas in an event of occlusion.13 Our patient had a haemorrhagic lesion of lower cervical cord, which is vulnerable due to complexity of the vascular supply and because cervical cord segments also have highest metabolic rates and greatest oxygen demands.14
In children, the different branches of subclavian artery supplying lower cervical cord are more prone to injury by minor trauma.15 In our patient, while there is no gross perinatal trauma to the spinal cord, minor trauma to the neck from intubation cannot be excluded. The MRI scan did not have evidence of traction injury. In our patient, haemorrhage at level of C7–T1 was an obvious trigger of clinical features of flaccid paralysis. Aspirin is commonly used in neonates for prophylaxis for thromboembolism but not reported to cause spinal haemorrhage. She also had multiple previously reported risk factors for cord ischaemia10 like thromboembolic phenomenon, reduced oxygenation, low cardiac output, vasospasm, aortic turbulence and umbilical catheterisation.
This case highlights various pathogenic mechanisms that led to early acquired SCI. To what extent the described anatomical peculiarities, the inherent metabolic properties or the mechanical vulnerability of the supplying arteries contribute to the predominantly cervical localisation of spinal cord haemorrhage and subsequent atrophy remains a matter of speculation. This is the first case in our knowledge that shows early onset of extensive spinal cord atrophy from haemorrhagic aetiology.
Conclusions
In an acutely unwell term infant with symptoms of flaccid paralysis from spinal cord damage, haemorrhagic aetiology needs to be considered in the differential diagnosis. To our knowledge, this is the first reported case of SCI in a term infant with a predominant haemorrhagic pathogenesis.16
References
Vialle R, Piétin-Vialle C, Ilharreborde B, Dauger S, Vinchon M, Glorion C . Spinal cord injuries at birth: a multicenter review of nine cases. J Matern Fetal Neonatal Med 2007; 20: 435–440.
Haldeman S, Fowler GW, Ashwal S, Schneider S . Acute flaccid neonatal paraplegia: a case report. Neurology 1983; 33: 93–95.
Aziz EM, Robertson AF . Paraplegia: complication of umblical artery catheterisation. J Pediatr 1973; 82: 1051–1052.
Brown MS, Phibbs RH . Spinal cord injury in newborns from the use of umblical artery catheters. J Perinatol 1988; 8: 105–110.
Clancy RR, Sladk JT, Rouke LB . Hypoxic-ischemic spinal cord injury following perinatal asphyxia. Ann Neurol 1989; 25: 185–189.
Rehan VK, Seshia MMK . Spinal cord birth injury-diagnostic difficulties. Arch Dis Child 1993; 69: 92–94.
Schneider H, Ballowitz L, Schachinger H, Hanefeld F, Dröszus JU . Anoxic encephalopathy with predominant involvement of basal ganglia, brain stem and spinal cord in perinatal period. Acta Neuropathol 1975; 32: 287–298.
Sladky R, Rorke LB . Perinatal hypoxic/ischemic spinal cord injury. Pediatr Pathol 1986; 6: 87–101.
Singer R, Joseph K, Gilai AN, Meyer S . Nontraumatic, acute neonatal paraplegia. J Pediatr Orthop 1991; 11: 588–593.
Arendar G, Samara E, Palmas C . Neonatal acquired paraplegia: retrospective review of 30 patients. J Pediatr Orthop B 1999; 8: 80–83.
Gilian LA . The arterial blood supply of the human spinal cord. J Comp Neurol 1958; 110: 75–103.
Adamkiewicz A . Die Blutgefasse des mennschlichen Ruckenmarkes. Sitzungsber Kaiserl Akad Wissensch Wien math- naturw CI III. Abt.1881; 83:469–502; 1882; 85:101–130.
Laguna J, Cravioto H . Spinal cord infarction secondary to occlusion of the anterior spinal artery. Arch Neurol 1973; 29: 134.
Duggal N, Lach B . Selective vulnerability of the lumbosacral spinal cord after cardiac arrest and hypotension. Stroke 2002; 33: 116–121.
Ahmann PA, Smith SA, Schwartz JF, Clark DB . Spinal cord infarction due to minor trauma in children. Neurology 1975; 25: 301–307.
Brown MS, Phibbs RH . Spinal cord injury in newborns from use of umbilical artery catheters: report of two cases and a review of the literature. J Perinatol 1988; 8: 105–110.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kulshrestha, R., Chowdhury, J., Lalam, R. et al. Spinal cord infarction in a sick neonate from predominant haemorrhagic aetiology: a case report. Spinal Cord Ser Cases 3, 17038 (2017). https://doi.org/10.1038/scsandc.2017.38
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/scsandc.2017.38
This article is cited by
-
Hypoxic Ischemic Encephalopathy with Cervical Spinal Cord Injury: A Diagnostic Dilemma
Indian Journal of Pediatrics (2024)