Abstract
Introduction:
We report the cases of a 68-year-old male with a filum terminale arteriovenous fistula (AVF) who was initially misdiagnosed with neuromyelitis optica spectrum disorder (NMOSD) based on imaging findings and false-positive aquaporin-4 IgG (AQP4-IgG).
Case presentation:
A 68-year-old male presented with slowly progressive weakness and numbness in his bilateral lower extremities. He was initially diagnosed with NMOSD and treated with immunosuppressive therapy based on findings of extensive spinal cord edema on magnetic resonance imaging (MRI) and initial negative spinal angiography as well as positive AQP4-IgG. Despite immunosuppressive treatment, his symptoms progressed slowly. He repeated MRI that showed persistent abnormal signal within the spinal cord. Second spinal angiography revealed filum terminale dural arteriovenous fistula (AVF). Finally, he underwent surgical disconnection of the fistula. Repeated AQP-IgG was reported negative.
Discussion:
Although NMOSD and spinal AVFs can share imaging findings on spinal cord MRI, typical clinical features of each disorder are distinct. Identification of AQP4-IgG is the hallmark to confirm a clinical diagnosis of NMOSD; however, different assays can vary in sensitivity and specificity. Although it is rare, false positives can occur especially at low titers. A misdiagnosis of NMOSD and delayed diagnosis of spinal AVF had significant clinical implications because the treatment of spinal AVF is surgical disconnection or endovascular embolization, whereas the treatment for NMOSD includes long-term immunosuppressive therapy. Clinicians should be aware of the potential technical issues in detecting AQP4-IgG especially in the context of patients with atypical presentations for NMOSD.
Similar content being viewed by others
Introduction
Filum terminale arteriovenous fistulas (AVFs) are a rare subtype of spinal vascular malformation with a single-hole direct communication between a continuation of the anterior spinal artery and a draining intraspinal vein.1 Clinical manifestations include the following: progressive motor weakness, sensory dysfunction and bowel and/or bladder symptoms due to venous congestive myelopathy, similar to the more common spinal dural AVFs.
Neuromyelitis optica spectrum disorder (NMOSD) is an inflammatory disorder of the central nervous system in which optic neuritis and longitudinal extensive transverse myelitis are major clinical features. Serum antibodies that target the water channel aquaporin-4 IgG (AQP4-IgG) are highly specific for the diagnosis of NMOSD.2 Hence, the incorporation of AQP4-IgG has become standard in NMOSD diagnostic criteria.
We report the case of a filum terminale AVF patient, who was initially misdiagnosed with NMOSD based on spinal cord edema, initial negative angiography and false-positive AQP4-IgG.
Case presentation
A 68-year-old Caucasian male presented with slowly progressive weakness and numbness in his bilateral lower extremities as well as shooting pain extending to both feet over 2 years. He had no bowel bladder incontinence. His initial MRI scan showed a long segmental intramedullary T2 hyperintense signal extending from T7 to conus medullaris and abnormal vascularity. He subsequently underwent spinal digital subtraction angiography (DSA) at another institution, which was reported negative, and he underwent extensive work-up for other causes of longitudinally extensive transverse myelitis. CSF study showed that the white cell count and red cell count were 1 and 3×106 per liter, respectively. CSF protein was 522 mg l−1 and CSF glucose was 3.0 mmol l−1. No oligoconal bands were found in either CSF or serum. His serum AQP4-IgG was positive (5.2 kU l−1; ELISA). He had no visual symptoms. The visual evoked potentials were normal. A diagnosis of NMOSD was made, and he was placed on Azathioprine (Imuran, Prometheus Laboratories Inc., San Diego, CA, USA).
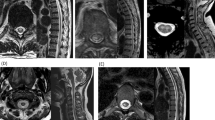
Over the following year, despite being on immunosuppressive therapy for presumed NMOSD, his lower extremity weakness slowly progressed and he was referred to our institution. His second MRI scan showed persistent abnormal T2 high signal with patchy enhancement along the lower thoracic cord as well as persistent abnormal enhancing flow voids (Figures 1a and b), raising the possiblity of a culprit vascular lesion. Spinal DSA was repeated and demonstrated a filum terminale AVF supplied by artery of Adamkiewicz arising at left T10 level with a point of fistulization at L4–L5 level (Figures 1c and d).
Pretreatment MR imaging, diagnostic spinal digital subtraction angiography (DSA) and follow-up post-treatment MR imaging of the patient. (a) Sagittal T2-weighted and (b) T1-weighted post-gadolinium MRI of the thoracic spine showed intramedullary T2 hyperintense signal extending from T7 to conus medullaris with patchy enhancement as well as abnormal perimedullary vessels (black arrow). (c) Early-phase and (d) late-phase spinal DSA showing enlarged Artery of Adamkiewicz (white arrow) with fistulization (white arrowhead) into draining spinal vein (black arrowhead) and perimedullary veins around the conus medullaris (black arrow). (e) Sagittal T2-weighted MRI of the thoracic spine at 2 months post surgical disconnection shows significant improvement of T2 hyperintense signal extending from T9 to conus medullaris.
He was treated with surgical disconnection, and no residual shunt was seen on the follow-up DSA. Repeat AQP4-IgG test was negative (<1.6 kU l−1; ELISA). He was gradually tapered off immunosuppressive therapy. On clinical follow-up, he continued to demonstrate gradual improvement in the weakness and sensory symptoms in his lower limbs and significant improvement in the intramedullary T2 hyperintensity (extending from T10 to conus medullaris) on his follow-up MRI (Figure 1e).
Discussion
Although NMOSD and spinal dural AVFs can share imaging findings on spinal cord MRI, typical clinical features of each disorder are distinct. NMOSD often presents with subacute onset of motor and sensory symptoms in the limbs that can improve with immunosuppressive treatment, whereas filum terminale and spinal dural AVFs usually present with slowly progressive onset of neurological symptoms that only improve with correction of the vascular lesion.
Spinal MRI features that can be observed in both NMOSD and spinal cord venous congestion from spinal AVFs include longitudinally extensive high T2 signal involving the spinal cord. However, evidence of abnormal perimedullary vessels should favor a diagnosis of spinal vascular malformation that requires spinal DSA for a definitive diagnosis.
According to the latest revision of NMOSD criteria,2 identification of AQP4-IgG has become the hallmark to confirm a clinical diagnosis; however, different assays can vary in sensitivity and specificity.2,3,4 Two commercially available assays are cell-based assays (CBAs) and ELISAs. CBAs are considered the most accurate techniques2,3,4 (mean sensitivity 76 and 0.1% false-positive rate2,5), and they have been recommended by the international panel on NMO diagnosis.2 ELISAs are more widely available and more commonly used, but they have a lower sensitivity (mean sensitivity 63–64%) and specificity (0.5–1.3%2,5) than CBAs. With both assays, false positives, when they occur, tend to occur at low titers, as in our case.2
Seroconversion from seropositive to seronegative AQP4-IgG status is possible in NMOSD patients treated with immunosuppressive therapy;6 therefore, the observed negative APQ4-IgG test after treatment in this case does not completely prove that the initial test was a false positive. However, the lack of response to immunosuppressive medication, followed by clinical and radiological improvement after surgical treatment strongly suggests that the initial antibody test was a false positive.
In our patient, the combination of an initial false-negative spinal angiogram and false-positive AQP4-IgG led to a misdiagnosis of NMOSD and delayed diagnosis of AVF. This has significant clinical implications because the treatment of AVF is surgical disconnection or endovascular embolization, whereas the treatment for NMOSD includes long-term immunosuppressive therapy with its potential attendant complications. Fortunately, our patient continues to show clinical improvement following surgical disconnection of his spinal AVF.
This case highlights a number of important points. First, it illustrates that when the clinical and radiological suspicion for a spinal vascular lesion is high, an initial negative DSA does not rule out spinal AVF, and if a repeat MRI remains suspicious, a repeat DSA should be performed. Second, this case underscores the fact that false-positive AQP4-IgG can be seen, although it is rare. Clinicians should be aware of the potential technical issues in detecting AQP4-IgG especially in the context of patients with atypical presentations for NMOSD and low-positive titer by ELISA. When in doubt, confirmatory AQP4-IgG testing using CBA should be performed, and further investigations should be considered to exclude alternative diagnoses.
References
Lim SM, Choi IS, David CA . Spinal arteriovenous fistulas of the filum terminale. AJNR Am J Neuroradiol 2011; 32: 1846–1850.
Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015; 85: 177–189.
Waters P, Reindl M, Saiz A, Schanda K, Tuller F, Kral V et al. Multicentre comparison of a diagnostic assay: aquaporin-4 antibodies in neuromyelitis optica. J Neurol Neurosurg Psychiatry 2016; 87: 1005–1015.
Waters PJ, McKeon A, Leite MI, Rajasekharan S, Lennon VA, Villalobos A et al. Serologic diagnosis of NMO: a multicenter comparison of aquaporin-4-IgG assays. Neurology 2012; 78: 665–671.
Pittock SJ, Lennon VA, Bakshi N, Shen L, McKeon A, Quach H et al. Seroprevalence of aquaporin-4-IgG in a northern California population representative cohort of multiple sclerosis. JAMA Neurol 2014; 71: 1433–1436.
Jasiak-Zatonska M, Kalinowska-Lyszczarz A, Michalak S, Kozubski W . The immunology of neuromyelitis optica-current knowledge, clinical implications, controversies and future perspectives. Int J Mol Sci 2016; 17: 273.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Suthiphosuwan, S., Oh, J. & Bharatha, A. Clinical pitfall: false-positive aquaporin-4 IgG leading to misdiagnosis of neuromyelitis optica spectrum disorder in patient with spinal arteriovenous fistula. Spinal Cord Ser Cases 3, 17030 (2017). https://doi.org/10.1038/scsandc.2017.30
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/scsandc.2017.30
This article is cited by
-
Foix-Alajouanine is another differential diagnosis in longitudinal myelitis thought to be a case of multiple sclerosis or neuromyelitis optica
Spinal Cord Series and Cases (2017)