Abstract
Introduction:
Direct intramedullary infections are considered very rare. Only few reports of Staphylococcus aureus myelitis have been published.
Case Presentation:
Our patient, a 79-year-old male, presented with a 2-day history of high-grade fever and high inflammatory markers and progressively developed tetraplegia during hospitalization. Lumbar puncture revealed cerebrospinal fluid pleocytosis and a spinal cord MRI revealed transverse myelitis at the level of C3–C5 and possible osteomyelitis of C5–T1. Two blood cultures were positive for methicillin-sensitive S. aureus. Despite control of the infection, there was no neurologic improvement.
Discussion:
The morbidity of infectious myelitis can be severe. Considering the rarity of S. aureus myelitis, experience gained from case reports is important. A brief review of the available literature is provided.
Similar content being viewed by others
Introduction
Acute transverse myelitis is a rare disease with a reported estimated annual incidence ranging from 1.34 to 6.2 per million.1 The morbidity of the disease is significant, with ~30–50% of affected patients having poor neurologic outcome.1,2,3,4 Back pain, rapid progression of symptoms and severe initial symptoms are poor prognostic factors.2,3,4 In many cases, a cause for the disease is not identified (idiopathic transverse myelitis).2,4 A history of antecedent infection is common.1 Diseases associated with secondary transverse myelitis include infections, systemic inflammatory/autoimmune diseases (for example, systemic lupus erythematosus, Sjogren syndrome, antiphospholipid syndrome and systemic sclerosis), neurosarcoidosis, demyelinating disorders (multiple sclerosis, neuromyelitis optica, ADEM) and paraneoplastic syndromes. Other causes of myelopathy that may mimic transverse myelitis clinically include compressive myelopathy, vascular diseases (for example, spinal cord infarct), radiation myelitis, trauma and nutritional deficiencies (for example, dorsal column degeneration with B12/vitamin E/copper deficiency).2,4,5
A long list of pathogens have been reported in association with acute myelitis.5 Typically, myelitis occurs after the resolution of the initial infection,5 which suggests an immune-mediated effect. Direct bacterial myelitis is considered very rare. Examples of pathogens associated with transverse myelitis are herpes viruses, enteroviruses, flaviviruses, HIV, mycoplasma, Borrelia burgdorferi and Mycobacterium tuberculosis. We found only few published case reports describing acute myelitis associated with Staphylococcus aureus infection, which are summarized later in the discussion section (Table 2).
Experience gained from case reports/series is important for the recognition and management of very rare diseases. Here we describe a case of acute transverse myelitis associated with S. aureus bacteremia and osteomyelitis.
Case report
A 79-year-old male presented to the emergency department with a 2-day history of high-grade fever, for which he was prescribed cefuroxime. He also mentioned an acute upper back and neck pain 5 days before, for which he had visited the on-call emergency department and for which he was prescribed analgesics. The patient reported a history of hypertension, dyslipidemia and hyperuricemia. His medications included valsartan/hydrochlorothiazide, lercanidipine, atorvastatin and allopurinol.
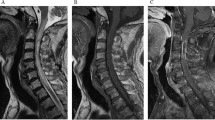
At presentation to the emergency department, a brief neurologic examination revealed left-arm weakness and ataxic broad-based walking. A brain CT was normal and lumbar puncture was performed. The cerebrospinal fluid (CSF) analysis revealed 298 white blood cells (58% lymphocytes), glucose 71 mg dl−1 (reference range 45–80 mg dl−1) (with a concurrent serum glucose of 160 mg dl−1) and total protein 1738 mg dl−1 (reference range 20–40 mg dl−1). Blood chemistry revealed a very high C-reactive protein (32 mg dl−1) (normal<0.5 mg dl−1). He was initially treated empirically for meningoencephalitis with a combination of intravenous dexamethasone, ceftriaxone, vancomycin, ampicillin and acyclovir pending blood cultures and CSF cultures and PCR. During the following 48 h, the patient progressively developed flaccid tetraplegia, a bilateral C3 sensory level and respiratory failure (shallow breathing and inefficient cough) all pointing to acute cervical spinal cord injury. The patient was fully oriented and cranial nerve functions were intact. MRI of the brain and spinal cord were performed and revealed increased T2 signal of the spinal cord at the level of C3–C5, findings compatible with transverse myelitis, and possible osteomyelitis of C5–T1 with inflammation of the perivertebral soft tissues (Figure 1). The brain MRI revealed bilateral nonspecific white matter lesions suggestive of chronic microvascular ischemic disease. The patient satisfied the previously described criteria for acute transverse myelitis and high-dose IV methylprednisolone (1 g daily) was started,6 but no response was seen. The CSF cultures and PCR were negative (see Table 1 for a summary of the investigations performed). The first two blood cultures came back positive for methicillin-sensitive S. aureus (MSSA). At day 4, after the above results were available, the initial empiric treatment was stopped and IV cloxacillin 2 g every 4 h was started and continued for 6 weeks.
MRI demonstrating cervical myelitis and osteomyelitis. Left: T2-weighted image depicting a swollen spinal cord with increased intramedullary signal (arrow). Right: T1-weighted image with gadolinium showing gadolinium enhancement of the spinal cord (asterisk) and of the vertebral bodies and prevertebral tissue (arrows).
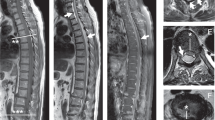
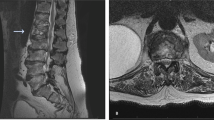
Abdominal ultrasound and transesophageal echocardiography did not reveal a source for the infection. Because of the lack of neurological improvement and suspicion of paraspinal abscess by a second MRI of the spinal cord (at day 10), neurosurgical consultation was obtained. Surgical drainage was attempted (at day 14). However, no abscess was found after surgical exploration of the area. Tissue from the inflammatory paravertebral space and affected bones was obtained. Culture of the tissue was negative. The biopsy of the bone was compatible with osteomyelitis. Despite control of the MSSA infection (as evidenced by resolution of fever, rapid blood culture sterilization, negative culture of the paravertebral tissue and fall of inflammatory markers), there was no neurological improvement. Two months later, the patient remained tetraplegic, although a follow-up MRI at 1 month showed significant reduction of the pathologic intramedullary signal. He died a few months later.
Discussion
We described a case of acute cervical myelitis associated with Staphylococcus aureus bacteremia and osteomyelitis. Despite control of the infection with antibiotics, our patients showed minimal neurological improvement.
Whether the myelitis was the result of direct Staphylococcus aureus intramedullary infection or the result of another pathogenetic mechanism is unclear. The detection of MSSA in two sets of blood cultures, the clinical presentation of acute high-grade fever and the resolution of fever and inflammatory markers with antibiotic treatment suggest with a high level of certainty that the patient truly had an MSSA infection. The presence of osteomyelitis (confirmed by imaging and biopsy) close to the site of myelitis suggests the possibility of contiguous spread of the infection, although the affected vertebrae (C5–T1) were at a lower level than the level of myelitis (C3–C5). On the other hand, MSSA was not isolated from CSF (both CSF cultures and PCR were negative) or culture of the tissue biopsies (which, however, were taken late in the course of antibiotic treatment), which points to alternative mechanisms (for example, parainfectious immune-mediated myelitis1,5).
Alternative causes of myelopathies were ruled out. Compressive myelopathy was excluded by MRI imaging and neurosurgical consultation and surgical exploration. A spinal cord infarct would have a more acute, rather than progressive, presentation and a vascular distribution of neurological findings on clinical examination (anterior spinal artery syndrome and posterior spinal artery syndrome), although atypical cases have been described. There was no evidence in favor of the systemic diseases associated with transverse myelitis. MRI imaging of the brain and spinal cord and the clinical presentation were not compatible with CNS demyelinating disorders. Findings from lab tests and imaging are summarized in Table 1.
Few cases of myelitis associated with S. aureus have been previously published.7,8,9, 10,11,12,13,14,15 Table 2 summarizes findings from previous case reports. Of note is the fact that S.aureus myelitis has been described even without contiguous spread (for example, ref 12). As in our case, in most other cases the cervical spinal cord was involved. High protein in the CSF was noted in all cases with available CSF analysis (>400 mg dl−1 in three of four cases). Our patient had a much higher CSF protein. Other causes of very high CSF protein include some cases of purulent meningitis, tuberculous meningitis, CNS tumors and blocked CSF flow (usually due to compression by tumors or abscess).16 All the above causes were ruled out in our patient (negative CSF PCR for tuberculosis, no suggestion of CNS tumor or abscess by imaging, negative CSF cytology). A block of the normal CSF flow due to severe spinal cord and meningeal swelling, and inflammation in combination with a herniated disk may explain the finding in our patient.
Our patient had a very poor neurologic outcome. Considering the rapid sterilization of blood cultures, the lack of isolation of MSSA from the prevertebral phlegmon, and the resolution of fever and inflammatory markers, the lack of improvement is unlikely to be due to antibiotic treatment failure, although inadequate eradication of an intramedullary focus of infection cannot be excluded. Potential explanations for the lack of response to antibiotics are the following: (1) Delayed initiation of appropriate antibiotic treatment with irreversible spinal cord damage. The patient had presented to the on-call emergency department 5 days before admission to our hospital. The only clues for infection from the available laboratory tests at that time was a neutrophilic predominance and severe eosinopenia (similar findings at presentation to our hospital). A CRP measurement was not available at the first visit; (2) our patient was much older than previously described successfully treated patients; (3) Immune-mediated transverse myelitis is possible, although there was no response to high-dose IV methylprednisolone; and (4) Cloxacillin has poor lipid solubility and CSF penetration may be insufficient.17 Furthermore, poor penetration of cloxacillin into abscesses of the central nervous system has been previously described.18 Failure of IV crystalline penicillin was also noted in a previous report of MSSA myelitis,12 supporting the hypothesis that penicillins may be a poor choice as monotherapy for bacterial myelitis. Nevertheless, successful treatment of MSSA central nervous system infections with high-dose cloxacillin has been described.19
In conclusion, myelitis associated with Staphylococcus aureus has been very rarely reported. Because of the potential significant morbidity of the disease associated with poor neurologic outcome, experience gained from case reports is important. Because of their poor CNS penetration, penicillins may not be an appropriate choice for monotherapy, as demonstrated by our case and a previous case of MSSA myelitis. Agents with better central nervous system penetration such as linezolid, levofloxacin and daptomycin,20 may be a better choice. Treatment regimens previously used successfully in S. aureus myelitis are shown in Table 2.
References
Berman M, Feldman S, Alter M, Zilber N, Kahana E . Acute transverse myelitis: incidence and etiologic considerations. Neurology 1981; 31: 966–971.
West TW, Hess C, Cree BA . Acute transverse myelitis: demyelinating, inflammatory, and infectious myelopathies. Semin Neurol 2012; 32: 97–113.
Kalita J, Misra UK, Mandal SK . Prognostic predictors of acute transverse myelitis. Acta Neurol Scand 1998; 98: 60–63.
de Seze J, Lanctin C, Lebrun C, Malikova I, Papeix C, Wiertlewski S et al. Idiopathic acute transverse myelitis: application of the recent diagnostic criteria. Neurology 2005; 65: 1950–1953.
Beh SC, Greenberg BM, Frohman T, Frohman EM . Transverse myelitis. Neurol Clin 2013; 31: 79–138.
Scott TF, Frohman EM, De Seze J, Gronseth GS, Weinshenker BG . Therapeutics, et al. Evidence-based guideline: clinical evaluation and treatment of transverse myelitis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2011; 77: 2128–2134.
Arendrup M, Scheibel JH . [Spondylitis and medullary transverse syndrome caused by Staphylococcus aureus]. Ugeskr Laeger 1997; 159: 7331–7334.
Eduwu J, Tabasam F, Bastidas AA, Dar K, Ahmed Y . Successful management of methicillin-resistant Staphylococcus aureus bacteremia complicated with diffuse myelitis. Infect Dis 2017; 49: 234–236.
He H, Jin L, Ju M, Tu G, Luo Z . Acute transverse myelitis of the cervical spine secondary to psoas abscess. BMC Infect Dis 2016; 16: 579.
Kulkarni GB, Pal PK, Veena Kumari HB, Goyal M, Kovoor JM, Nadig S et al. Community-acquired methicillin-resistant Staphylococcus aureus pyomyositis with myelitis: a rare occurrence with diverse presentation. Neurol India 2009; 57: 653–656.
Ruiz Ruiz FJ, Martin Lorenzo B, Amores Arriaga A, Ruiz Laiglesia FJ, Hualde Enguita AM, Perez Calvo JI . [Acute transverse myelopathy associated to S. aureus: a difficult differential diagnosis]. Neurologia 2004; 19: 130–133.
Saini M, Prasad K, Ling LM, Tan K . Transverse myelitis due to Staphylococcus aureus may occur without contiguous spread. Spinal Cord 2014; 52 (Suppl 2): S1–S2.
Kastenbauer S, Winkler F, Fesl G, Schiel X, Ostermann H, Yousry TA et al. Acute severe spinal cord dysfunction in bacterial meningitis in adults: MRI findings suggest extensive myelitis. Arch Neurol 2001; 58: 806–810.
Kalkan G, Karaoglu A, Vurucu S, Unay B, Gok F, Akin R et al. Methicillin resistant Staphylococcus aureus pneumonia accompanied with transverse myelitis. Eur J Gen Med 2012; 9: 203–204.
Friess HM, Wasenko JJ . MR of staphylococcal myelitis of the cervical spinal cord. AJNR Am J Neuroradiol 1997; 18: 455–458.
Jurado R, Walker HK . Cerebrospinal Fluid In: Walker HK, Hall WD, Hurst JW (eds) Clinical Methods: The History, Physical, and Laboratory Examinations 3rd edn. Butterworth: Boston, 1990.
Schievink HI, Mattie H, Thomeer RT, Van Strijen E . The passage of cloxacillin into cerebrospinal fluid in the absence of meningitis. Br J Clin Pharmacol 1993; 36: 57–60.
de Louvois J, Gortvai P, Hurley R . Antibiotic treatment of abscesses of the central nervous system. Br Med J 1977; 2: 985–987.
Fong IW . Staphylococcal central nervous system infections treated with cloxacillin. J Antimicrob Chemother 1983; 12: 607–612.
Nau R, Sorgel F, Eiffert H . Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev 2010; 23: 858–883.
Acknowledgements
We thank Dr K Psyllakis and Dr V Stylianaki of the Department of Radiology for providing the MRI images.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Karakonstantis, S., Galani, D., Maragou, S. et al. A rare case of acute transverse myelitis associated with Staphylococcus aureus bacteremia and osteomyelitis. Spinal Cord Ser Cases 3, 17029 (2017). https://doi.org/10.1038/scsandc.2017.29
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/scsandc.2017.29
This article is cited by
-
Acute transverse myelitis after SARS-CoV-2 infection: a rare complicated case of rapid onset paraplegia
Journal of NeuroVirology (2021)