Abstract
Study design:
Systemic review
Objective:
We carried out a systematic review and meta-analysis to assess the efficacy and safety of phosphodieterase-5 (PDE5) inhibitors on erectile dysfunction (ED) secondary to spinal cord injury (SCI).
Methods:
A literature review was performed to identify all published randomized, double-blind, placebo-controlled trials of PDE5 inhibitors for treatment of ED secondary to SCI. The search included the following database: MEDLINE, EMBASE and the Cochrane Library. The outcomes and complications analyzed involved the Global Efficacy Question (GEQ), sexual encounter profile diary question 2 and 3 (SEP2 and SEP3) and adverse events. All statistical analysis was performed using Stata 12.0 software (Stata Corp., College Station, TX, USA).
Results:
Six publications were used in analysis, including six randomized controlled trials that compared PDE5 inhibitors with placebo. Compared with placebo, PDE5 inhibitors were associated with significant improvements in GEQ (OR 11.997, 95% CI 8.073–17.830, P<0.0001), SEP2 (RR 1.847, 95% CI 1.561–2.185, P<0.0001) and SEP3 (RR 2.738, 95% CI 2.084–3.598, P<0.0001). Despite significant greater incidences of some adverse events observed (headache: RR 3.717, 95% CI 2.309–5.982, P<0.0001; flushing: RR 9.281, 95% CI 2.858–30.147, P<0.0001; gastrointestinal discomfort: RR 9.064, 95% CI 2.116–38.827, P=0.003), most adverse events were mild to moderate and transient.
Conclusions:
This systematic review and meta-analysis indicate that PDE5 inhibitors are effective and well tolerated to treat ED secondary to SCI compared with placebo, as measured by response to GEQ, SEP2, SEP3 and incidence of adverse events. PDE5 inhibitors could be considered as the first choice in the treatment of ED patients with SCI.
Similar content being viewed by others
Introduction
Approximately 11 000 new cases are estimated to suffer from spinal cord injury (SCI) in America each year and 60% of them are between 16 and 30 years of age. Moreover, men account for 82% of all patients with SCI.1, 2 Erectile dysfunction (ED) is a common condition in individuals with SCI.3 A high prevalence of ED is reported in about 80% of SCI patients.4 It is an especially bothersome condition in young men who usually had an active sexual activity before their accident.5
ED is defined as the persistent inability to achieve or maintain a penile erection for satisfactory sexual performance.6 Most men with SCI have some type of penile erection, but it is usually not hard enough or does not last long enough for sexual life.1 ED not only often results in disorder of self-esteem but also deteriorates partner relationship. Hence, ED has a negative effect on the life of men and their sexual partner.7 Health-care providers deem sex life significant in the process of rehabilitation after SCI and give high priority to improving sexual activity for subjects with SCI.8, 9
Nitric oxide (NO) released by nonadrenergic-noncholinergic nerves in the penis has an important role in initiating and maintaining of the penile erection process.10, 11 NO diffuses to smooth where it augments the formation of cyclic guanosine monophosphate, which acts as a second messenger for engorgement of penile vasculature and penile erection.12, 13 Phosphodieterase-5 (PDE5) could facilitate the breakdown of cyclic guanosine monophosphate in the cavernosal smooth muscle where PDE5 is sufficient.14 The PDE5 inhibitors function by inhibiting the catalytic site of PDE5, an enzyme that could hydrolyze cyclic guanosine monophosphate. As a result, with a corresponding increase in cavernosal tumescence and rigidity, smooth muscle relaxation is enhanced and blood inflow into the corpora cavernosa.6, 15 As the first oral selective phosphodieterase type5 (PDE5) inhibitor, the introduction of sildenafil in 1998 is an important advance in the treatment of ED.16 Two other PDE5 inhibitors, vardenafil and tadalafil, were also developed in recent years.17 The oral treatment with PDE5 inhibitors is considered the least invasive method.18 PDE5 inhibitors have also changed the treatment of ED in SCI patients, becoming the preferred treatment in subjects with SCI since the introduction of sildenafil because of their ease of use and effectiveness.19, 20, 21 Nevertheless the role of PDE5 inhibitors to treat ED caused by SCI has not been well assessed. It is still challenging to identify the efficacy and safety of PDE5 inhibitors in SCI patients suffering from ED. In this case, superior evidence-based medicine support is absent in treatment of ED in SCI subjects. Thus, we aimed to analyze highly selected quality randomized controlled trials (RCTs) with meta-analytic methodology and resolve question of the efficacy and safety of PDE5 inhibitors for ED secondary to SCI.
Materials and methods
Search strategy
The pertinent studies were obtained by searching Medline, Embase and Cochrane Library up to March 2015. Search keywords used were ED, SCI, phosphodieterase type 5 inhibitors, PDE5, PDE5-I, sildenafil, vardenafil, tadalafil, avanafil, udenafil and mirodenafil. Furthermore, the references of included trials and relevant review articles were searched to identify additional potentially pertinent studies. We did not apply any methodological search filters and date or language limits. Two reviewers independently employed this search strategy to screen all titles and abstracts for identifying eligible trials.
Inclusion criteria and trials selection
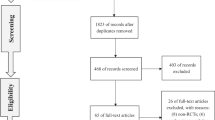
RCTs meeting the following criteria were included: (i) the study design involved treatment with PDE5 inhibitors; (ii) the accurate data were provided for analysis, including the total number of subjects and values of necessary outcomes like the Global Efficacy Question (GEQ), sexual encounter profile diary question 2 and 3 (SEP2 and SEP3) or incidences of adverse events; and (iii) the full-text of trial could be appraised. The most recent publication was adopted for meta-analysis if the same study was reported in different journals or years. We adopted each trial with multiple experiments conducted by the same group of searchers. We contacted authors if the data we need were all included in the articles. Two authors assessed literatures meeting the inclusion criteria independently and discrepancy was resolved by consensus with another author. A flow diagram of trial selection process is presented in Figure 1.
Quality assessment
The methodological quality of the included trials was appraised by two reviewers based on the Cochrane Collaboration Reviewers’ Handbook for Systemic Reviews of Interventions.22 The quality assessment of retrieved RCTs involved evaluation of sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other bias. The judgement ('Low risk ', 'High risk' or 'Unclear risk') is made for each entry. According to the quality assessment criteria, we assigned each trial to the three different quality categories. If all quality criteria were completely met and the study was considered to have a low risk of bias, we ranked it as class A; if one or more of quality criteria were not adequately met or were unclear and the trial was deemed to have a moderate risk of bias, we ranked it as class B; if one or more of the criteria were not included or not met and the trial was deemed to have a high risk of bias, we ranked it as class C. Discrepancy was resolved by consensus with a third reviewer.
Data extraction
Data extraction was performed independently by two reviewers with predesigned data extraction forms. The following information was extracted for each trial: (i) participants: diagnostic criteria, number of each group, age, severity of SCI, withdrawal and lost to follow-up; (ii) method: study design, randomized methods, blinding method and allocation concealment method; (iii) intervention: details of treatment, such as type, dose and treatment duration; (iv) outcomes: the response to the GEQ, the response to the SEP2 and SEP3 and all reported adverse effects; (v) other: country and publication year. Disagreements were resolved via consultation with another author.
Statistical analysis and meta-analysis
All statistical analysis was performed using Stata 12.0 software (Stata Corp., College Station, TX, USA). All data we extracted were dichotomous data; hence, we estimated the odds ratio (OR) or relative risk (RR) for outcomes and complications. A 95% confidence interval (95% CI) was used. We used the χ2 test and the I2 test to appraise statistical heterogeneity between included trials. An I2 statistic of <50% suggests that low heterogeneity exists among studies, an I2 statistic of 50–75% suggests that moderate heterogeneity exists, and I2 statistic of >75% suggests that high heterogeneity exists.23 If I2 statistic is more than 50%, it was deemed to have substantial statistical heterogeneity in included studies. Whether a fixed-effects model was used mainly depends on the results of the I2 test for heterogeneity. We chose a fixed-effect model for meta-analysis if the studies were homogeneous. Otherwise, a random-effect model was used and a sensitivity analysis was performed to explore the reliability of results. A subgroup analysis was performed simultaneously to explore possible heterogeneity by the types of PDE5 inhibitors, doses of drugs, duration of treatment and severity of ED if data were sufficient. In the case of excessive heterogeneity, we only performed descriptive analysis instead of meta-analysis.
Results
Study identification and selection
A total of 754 studies were selected in the initial database search, and 660 studies were excluded based on title and abstract of articles. Fifty-nine studies were excluded on account of duplicate, and 29 studies were excluded because they did not meet our inclusion criteria after reading full-text. In all, six articles24, 25, 26, 27, 28, 29 involving six RCTs were adopted for analysis: four RCTs that compared sildenafil with placebo, one RCT that compared vardenafil with placebo and one RCT that compared tadalafil with placebo. The baseline characteristics of the trials included in our meta-analysis are listed in Table 1.
Quality of the individual studies
All six RCTs were double blinded and the randomization process was described. We summarized the results of all the risk of bias assessment in Figure 2. The level of quality of the two trials was C, and the level of the rest was B.
(a) Risk of bias summary: review authors’ judgments about each risk of bias Item for each included study. (b) Risk of bias graph: review authors’ judgments about each risk of bias item presented as percentages across all included studies. A full color version of this figure is available at the Spinal Cord journal online.
Efficacy end points
Four trials reported the response to GEQ ('Has the treatment you have been taking during this study improved your penile erection’). A total of 366 patients and 261 patients were involved, respectively, in trials to compare the efficacy of PDE5 inhibitors versus placebo. The heterogeneity test indicated no significant heterogeneity (P=0.191; I2=36.9%), and hence the fixed-effect model was used. The pooled estimate of the OR was 11.997, the 95% CI was 8.073–17.830 and P<0.0001 (Figure 3). This result suggests that PDE5 inhibitors show statistically greater improvement in response to GEQ compared with placebo.
Only two trials contributed to SEP2 and SEP3 analysis. Trials comparing the efficacy of PDE5 inhibitors versus placebo involved 334 patients and 235 patients, respectively. Sexual Encounter Profile is deemed as a diary in which patients’ responses ('Yes/no') concerning specific aspects of sexual encounter were reported. The SEP2 asked 'Were you able to insert your penis into your partner’s vagina?', and the SEP3 asked 'Did your penile erection last long enough for you to have successful intercourse?' No heterogeneity for both questions was observed in pooled analysis (SEP2: P=0.948, I2=0.0%; SEP3: P=0.896, I2=0.0%), and hence the fixed-effect model was used. The pooled results showed that a much greater number of patients in the PDE5 inhibitor group to respond to SEP2 and SEP3 positively (SEP2: RR 1.847, 95% CI 1.561–2.185, P<0.0001; SEP3: RR 2.738, 95% CI 2.084–3.598, P<0.0001; Figure 4).
Adverse events
Adverse events of PDE5 inhibitors and relevant data were reported in five trials. Studies comparing adverse events of PDE5 inhibitors versus placebo included 590 patients and 479 patients, respectively. The individual adverse events such as headache, flushing and gastrointestinal discomfort were analyzed (Table 2). Nevertheless, flushing, one of the adverse events, was only mentioned in three articles. No significant heterogeneity for incidence of headache, flushing and gastrointestinal discomfort was found in the pooled analysis (headache: P=0.784, I2=0.0%; flushing: P=0.652, I2=0.0%; gastrointestinal discomfort: P=0.915, I2=0.0%), and hence the fixed-effect model was used. Incidence of headache, flushing and gastrointestinal discomfort showed significant high incidence in the PDE5 Inhibitor group (headache: RR 3.717, 95% CI 2.309–5.982, P<0.0001; flushing: RR 9.281, 95% CI 2.858–30.147, P<0.0001; gastrointestinal discomfort: RR 9.064, 95% CI 2.116–38.827, P=0.003; Figure 5). However, most adverse events were mild to moderate and transient.
(a) Forest plot diagram showing (a) headache incidence (P=0.784, I2=0.0%, RR 3.717, 95% CI 2.309–5.982), (b) flushing incidence (P=0.652, I2=0.0%, RR 9.281, 95% CI 2.858–30.147) and (c) gastrointestinal discomfort incidence (P=0.915, I2=0.0%, RR 9.064, 95% CI 2.116–38.827). A full color version of this figure is available at the Spinal Cord journal online.
Publication bias
Visual inspection of the funnel plot for response to GEQ and incidence of headache and gastrointestinal discomfort found a slightly asymmetrical distribution. However, according to Begg’s and Egger’s test, no evidence of substantial publication bias existed among the included trials for response to GEQ (Begg’s test, P=1.000; Egger’s test, P=0.610; Figure 6), response to SEP2 (Begg’s test, P=1.000), response to SEP3 (Begg’s test, P=1.000), incidence of headache (Begg’s test, P=0.462; Egger’s test, P=0.626, Figure 7), incidence of flushing (Begg’s test, P=1.000; Egger’s test, P=0.509) and incidence of gastrointestinal discomfort (Begg’s test, P=0.089; Egger’s test, P=0.073).
Discussion
To our knowledge, this is first meta-analysis with RCTs to assess the efficacy and safety of PDE5 inhibitors for ED caused by SCI. A recent publication by Nicole Rizio reviewed the medical literature in 2000–2010. Nicole Rizio considered that sildenafil, tadalafil and vardenafil have proved to be comparably efficacious in the treatment of ED in men with SCI, as all studies included had shown a statistical increase in the international index of erectile function scores.30 Nevertheless, this review did not employ a meta-analysis methodology.
Most patients with SCI require some supportive treatment for their ED because their penile erections are often less rigid and have more difficulty to maintain.31, 32 A variety of management options could be adopted for individuals with SCI.33 However, therapies for ED caused by SCI remain challenging, and PDE5 inhibitors, vacuum devices, penile implants, intraurethral prostaglandins, percutaneous perineal electrostimulation, transdermal nitroglycerin, intracavernous injection of papaverine and some other therapies are still under debate.33, 34, 35, 36, 37, 38, 39
The aim of this systematic review focused on comprehensively appraising efficacy and safety of PDE5 inhibitors in patients with SCI. In the present review, a large portion of ED patients secondary to SCI greatly benefited from PDE5 inhibitors (sildenafil, vardenafil or tadalafil). Overall, patients receiving PDE5 inhibitors had a higher percentage of positive response to GEQ, compared with men allocated to the placebos groups. In addition, significant improvements were found in key secondary efficacy end points of SEP2 and SEP3 for PDE5 inhibitor treatment groups. Therefore, our results show that PDE5 inhibitors could significantly improve erectile function in SCI patients compared with placebo. All researchers instructed patients to take PDE5 inhibitors as needed approximately 1 h or at least 45 min prior to sexual activity, and no trial using once-a-day administration was included. Whether once-a-day administration of PDE5 inhibitors could improve penile erection is still obscure, and new trials are needed to address this issue.
Safety data from the trials in this meta-analysis suggest that PDE5 inhibitor administration was generally well tolerated. Despite significant greater incidences of some adverse events observed, most adverse events were mild to moderate and transient. The most commonly reported adverse events were headache, flushing and gastrointestinal discomfort. Only four cases of discontinuation due to serious adverse events were reported in about 600 men receiving PDE5 inhibitors included in this review. Moreover, it is reported in a study that the frequency of these PDE5 inhibitor-characteristic adverse events declined over the course of study.28 A majority of included RCTs suggested no clinically significant changes in heart rate, blood pressure and laboratory tests in the PDE5 inhibitors groups.
Information on response of GEQ was primarily based on four RCTs,25, 27, 28, 29 and the data on response of SEP were collected from two RCTs,28, 29 and the data on incidence of adverse events synthesized from five articles.24, 25, 26, 28, 29 The reason why we did not analyze efficacy of PDE5 inhibitors with international index of erectile function was that they did not report values of standard deviation, although it has been deemed to have a high degree of sensitivity and specificity for extrapolating treatment-related changes in the erectile function.40
The data in studies included in this meta-analysis all derived from randomized, double-blind, placebo-controlled trials. On the basis of the quality assessment scale developed, the quality of the individual trials in the meta-analysis was conforming. The results of this review acquire great importance from everyday clinical practice. However, some limitations of the present results should be mentioned. First, we did not comment on the differences in efficacy and safety among three PDE5 inhibitors (sildenafil, vardenafil and tadalafil) because no direct comparisons were made in these six trials. Second, as the longest duration of the therapy was 24 weeks, long-term efficacy and safety of PED5 inhibitors cannot be detected. Third, only six RCTs with a limited number of patients assessing the efficacy and safety of PDE5 inhibitors and the data from unpublished trials were not included in the analysis. In addition, we did not determine whether the characteristics of patients are potential risk factors (age, body mass index and other baseline parameters) that could influence the results. Finally, the efficacy of daily treatment with PDE5 inhibitors was not assessed because of a lack of relevant studies. Futher high-quality trials with larger samples from all over the world are proposed to learn more about the efficacy and safety of the therapies for ED secondary to SCI.
Conclusions
This systematic review and meta-analysis indicate that PDE5 inhibitors are effective to treat ED secondary to SCI compared with placebo, as measured by response to GEQ, SEP2 and SEP3. PDE5 inhibitors can be deemed as the first choice in the treatment of ED patients with SCI. In addition, adverse events were transient and serious adverse events were few, which showed that PDE5 inhibitors were well tolerated. Further high-quality studies are expected to evaluate the long-term outcomes, relationship between the outcomes and characteristic of patients and efficacy of once daily PDE5 inhibitors in treatment of ED secondary to SCI.
References
Derry F, Hultling C, Seftel AD, Sipski ML . Efficacy and safety of sildenafil citrate (Viagra) in men with erectile dysfunction and spinal cord injury: a review. Urology 2002; 60 (Suppl 2): 49–57.
Gans WH, Zaslau S, Wheeler S, Galea G, Vapnek JM . Efficacy and safety of oral sildenafil in men with erectile dysfunction and spinal cord injury. J Spinal Cord Med 2001; 24: 35–40.
Strebel RT, Reitz A, Tenti G, Curt A, Hauri D, Schurch B . Apomorphine sublingual as primary or secondary treatment for erectile dysfunction in patients with spinal cord injury. BJU Int 2004; 93: 100–104.
Lombardi G, Nelli F, Celso M, Mencarini M, Del Popolo G . Treating erectile dysfunction and central neurological diseases with oral phosphodiesterase type 5 inhibitors. Review of the literature. J Sex Med 2012; 9: 970–985.
Althof SE, Levine SB . Clinical approach to the sexuality of patients with spinal cord injury. Urol Clin North Am 1993; 20: 527–534.
Goldstein I, Lue TF, Padma-Nathan H, Rosen RC, Steers WD, Wicker PA . Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. N Engl J Med 1998; 338: 1397–1404.
Althof SE . Quality of life and erectile dysfunction. Urology 2002; 59: 803–810.
Fisher TL, Laud PW, Byfield MG, Brown TT, Hayat MJ, Fiedler IG . Sexual health after spinal cord injury: a longitudinal study. Arch Phys Med Rehabil 2002; 83: 1043–1051.
Pervin-Dixon L . Sexuality and the spinal cord injured. J Psychosoc Nurs Mental Health Serv 1988; 26: 31–34.
Carson CC . Erectile dysfunction: evaluation and new treatment options. Psychosom Med 2004; 66: 664–671.
Hamidi Madani A, Asadolahzade A, Mokhtari G, Shahrokhi Damavand R, Farzan A, Esmaeili S . Assessment of the efficacy of combination therapy with folic acid and tadalafil for the management of erectile dysfunction in men with type 2 diabetes mellitus. J Sex Med 2013; 10: 1146–1150.
Hedlund P, Aszodi A, Pfeifer A, Alm P, Hofmann F, Ahmad M et al. Erectile dysfunction in cyclic GMP-dependent kinase I-deficient mice. Proc Natl Acad Sci USA 2000; 97: 2349–2354.
Burnett AL . Role of nitric oxide in the physiology of erection. Biol Reprod 1995; 52: 485–489.
Boolell M, Allen MJ, Ballard SA, Gepi-Attee S, Muirhead GJ, Naylor AM et al. Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int J Impot Res 1996; 8: 47–52.
Yang L, Qian S, Liu L, Pu C, Yuan H, Han P et al. Phosphodiesterase-5 inhibitors could be efficacious in the treatment of erectile dysfunction after radiotherapy for prostate cancer: a systematic review and meta-analysis. Urol Int 2013; 90: 339–347.
Martin AL, Huelin R, Wilson D, Foster TS, Mould JF . A systematic review assessing the economic impact of sildenafil citrate (Viagra) in the treatment of erectile dysfunction. J Sex Med 2013; 10: 1389–1400.
Campbell HE . Clinical monograph for drug formulary review: erectile dysfunction agents. J Manag Care Pharm 2005; 11: 151–171.
Fode M, Krogh-Jespersen S, Brackett NL, Ohl DA, Lynne CM, Sonksen J . Male sexual dysfunction and infertility associated with neurological disorders. Asian J Androl 2012; 14: 61–68.
Sanchez Ramos A, Vidal J, Jauregui ML, Barrera M, Recio C, Giner M et al. Efficacy, safety and predictive factors of therapeutic success with sildenafil for erectile dysfunction in patients with different spinal cord injuries. Spinal Cord 2001; 39: 637–643.
Kimoto Y, Sakamoto S, Fujikawa K, Tachibana T, Yamamoto N, Otani T . Up-titration of vardena fi l dose from 10 mg to 20 mg improved erectile function in men with spinal cord injury. Int J Urol 2006; 13: 1428–1433.
Del Popolo G, Li Marzi V, Mondaini N, Lombardi G . Time/duration effectiveness of sildenafil versus tadalafil in the treatment of erectile dysfunction in male spinal cord-injured patients. Spinal Cord 2004; 42: 643–648.
Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [ updated March 2011]. The Cochrane Collaboration 2011. Available at http://handbook.cochrane.org/.
Higgins JP, Thompson SG, Deeks JJ, Altman DG . Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560.
Khorrami MH, Javid A, Moshtaghi D, Nourimahdavi K, Mortazavi A, Zia HR . Sildenafil efficacy in erectile dysfunction secondary to spinal cord injury depends on the level of cord injuries. Int J Androl 2010; 33: 861–864.
Maytom MC, Derry FA, Dinsmore WW, Glass CA, Smith MD, Orr M et al. A two-part pilot study of sildenafil (VIAGRA) in men with erectile dysfunction caused by spinal cord injury. Spinal Cord 1999; 37: 110–116.
Giuliano F, Hultling C, El Masry WS, Smith MD, Osterloh IH, Orr M et al. Randomized trial of sildenafil for the treatment of erectile dysfunction in spinal cord injury. Sildenafil Study Group. Ann Neurol 1999; 46: 15–21.
Ergin S, Gunduz B, Ugurlu H, Sivrioglu K, Oncel S, Gok H et al. A placebo-controlled, multicenter, randomized, double-blind, flexible-dose, two-way crossover study to evaluate the efficacy and safety of sildenafil in men with traumatic spinal cord injury and erectile dysfunction. J Spinal Cord Med 2008; 31: 522–531.
Giuliano F, Rubio-Aurioles E, Kennelly M, Montorsi F, Kim ED, Finkbeiner AE et al. Efficacy and safety of vardenafil in men with erectile dysfunction caused by spinal cord injury. Neurology 2006; 66: 210–216.
Giuliano F, Sanchez-Ramos A, Lochner-Ernst D, Del Popolo G, Cruz N, Leriche A et al. Efficacy and safety of tadalafil in men with erectile dysfunction following spinal cord injury. Arch Neurol 2007; 64: 1584–1592.
Rizio N, Tran C, Sorenson M . Efficacy and satisfaction rates of oral PDE5is in the treatment of erectile dysfunction secondary to spinal cord injury: a review of literature. J Spinal Cord Med 2012; 35: 219–228.
Biering-Sorensen F, Sonksen J . Sexual function in spinal cord lesioned men. Spinal Cord 2001; 39: 455–470.
Courtois FJ, Charvier KF, Leriche A, Raymond DP, Eyssette M . Clinical approach to erectile dysfunction in spinal cord injured men. A review of clinical and experimental data. Paraplegia 1995; 33: 628–635.
Linsenmeyer TA . Treatment of erectile dysfunction following spinal cord injury. Curr Urol Rep 2009; 10: 478–484.
Sidi AA, Cameron JS, Dykstra DD, Reinberg Y, Lange PH . Vasoactive intracavernous pharmacotherapy for the treatment of erectile impotence in men with spinal cord injury. J Urol 1987; 138: 539–542.
Tang SF, Chu NK, Wong MK . Intracavernous injection of prostaglandin E1 in spinal cord injured patients with erectile dysfunction. A preliminary report. Paraplegia 1995; 33: 731–733.
Renganathan R, Suranjan B, Kurien T . Comparison of transdermal nitroglycerin and intracavernous injection of papaverine in the treatment of erectile dysfunction in patients with spinal cord lesions. Spinal Cord 1997; 35: 99–103.
Bodner DR, Haas CA, Krueger B, Seftel AD . Intraurethral alprostadil for treatment of erectile dysfunction in patients with spinal cord injury. Urology 1999; 53: 199–202.
Soler JM, Previnaire JG, Denys P, Chartier-Kastler E . Phosphodiesterase inhibitors in the treatment of erectile dysfunction in spinal cord-injured men. Spinal Cord 2007; 45: 169–173.
Shafik A, Shafik AA, Shafik IA, El Sibai O . Percutaneous perineal electrostimulation induces erection: clinical significance in patients with spinal cord injury and erectile dysfunction. J Spinal Cord Med 2008; 31: 40–43.
Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A . The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997; 49: 822–830.
Acknowledgements
This program is supported by young leader funded projects of colleges and universities in Shanxi Province (JINKEJIAO [2012]10).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Jia, DD., Shuang, WB., Cheng, T. et al. Efficacy and safety of phosphodieterase-5 inhibitors for treatment of erectile dysfunction secondary to spinal cord injury: a systemic review and meta-analysis. Spinal Cord 54, 494–501 (2016). https://doi.org/10.1038/sc.2016.3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2016.3
This article is cited by
-
Infection rate of penile prosthesis implants in men with spinal cord injury: a meta-analysis of available evidence
International Journal of Impotence Research (2022)
-
Erectile Dysfunction and Neurological Comorbidities: a Contemporary Review
Current Sexual Health Reports (2020)
-
Reply to ‘Efficacy and safety of phosphodiesterase-5 inhibitors for treatment of erectile dysfunction secondary to spinal cord injury: a systemic review and meta-analysis’
Spinal Cord Series and Cases (2017)