Abstract
Study design:
A prospective cohort with acute tetraplegia.
Objective:
The purpose of this study was to investigate acute changes in serum brain-derived neurotrophic factor (BDNF) concentrations in tetraplegic spinal cord-injured (SCI) athletes during a typical training session of wheelchair rugby.
Settings:
German Sport University Cologne, Cologne, Germany.
Methods:
Eleven male SCI (AIS A and B) athletes completed a 90-min training session: The warm-up period included continuous pushing, submaximal increasing sprints and agility drills. The main training section comprised ball handling, passing drills, scrimmage activity and tactical practice. At the end of the training session, the athletes did moderate continuous pushing as a short cool-down. Venous blood samples were taken at rest before exercise, after the warm-up period and immediately following the first part of the main training section. Serum was pipetted after 30 min of blood sample resting and a subsequent centrifugation. BDNF concentrations were measured using an enzyme immunoassay ELISA kit.
Results:
Heart rate (P<0.01) and lactate (P=0.04 and P<0.01) concentration differed significantly in warm-up and main training part in comparison with basal values at rest. At rest, BDNF concentrations were 33.2±21.6 ng ml−1, after warm up 31.9±18.9 ng ml−1 and after the training session 29.9±11 ng ml−1, without significant differences (P>0.05).
Conclusions:
A typical wheelchair rugby training session does not affect basal serum BDNF concentration in elite SCI athletes. In comparison with concentrations previously reported in healthy subjects, the current values at rest were slightly higher or rather at the upper limit.
Similar content being viewed by others
Introduction
Regular or single bouts of physical activity, such as aerobic endurance exercise or strength training, are associated with manifold positive effects for able-bodied people.1 Beside an improved preventive cardiorespiratory fitness and muscular capacity, associated with a reduced risk of cardiovascular diseases, mental health and memory performance are increased as well.2 In this context, neurotrophic factors such as the brain-derived neurotrophic factor (BDNF) have a vital role in terms of neurogenesis, neuroplasticity and nerve protection.3 Regular physical exercise maintains higher basal BDNF levels and the reaction to exercise is stronger than with other neurotrophins like NGF (neurotrophic growth factor) or NT (neurotrophin) 3-5.2 The effect of endurance and weight training on BDNF concentration in blood plasma differs.2, 4 Intensity, duration and type of endurance exercise lead to different BDNF concentrations in blood serum5, 6 and even a singular sodium lactate infusion raised serum BDNF levels.7
In animals with a SCI, exercise leads to higher concentrations of neurotrophic factors like BDNF.8, 9 These modulations might have an impact on the survival of motoneurons, sprouting and synaptic remodelling of injured axons. Local injections of adeno-associated virus constructs or fibroblasts implying BDNF allows rats with complete paraplegia spinal cord transection injuries to reorganize locomotor skills.10, 11 In addition, BDNF concentration is immediately increased after experimentally induced injury in the rat spinal cord.11, 12, 13
There is strong evidence that in depression, Alzheimer's and multiple sclerosis, basal concentrations of BDNF are decreased. To raise circulating BDNF levels, exercise has been applied in several neuronal disorders like depression,14, 15 schizophrenia16 and neurodegenerative diseases like Alzheimer's17 as well as multiple sclerosis.18 Depending on lesion levels in tetra- and paraplegia SCI, there are major influences on central nerve system and peripheral nerve system. Impairments in terms of muscle functions or organ and hormonal regulation may also trigger different reactions of neurotrophic factors like BDNF.19
To the best of our knowledge, there is only one study that examines BDNF levels in spinal cord injury (SCI) subjects.20 Rojas and colleagues20 demonstrate higher basal BDNF values (37.2±19.8 ng l−1) for paraplegic SCI athletes than previously reported in able-bodied subjects. BDNF in paraplegic athletes increases after short (10 min) moderate hand cycling activity but not after a following marathon race simulation. Wheelchair rugby, an attractive sport for athletes with a tetraplegical SCI, is well analyzed in respect of performance, testing and analytics of energy expenditure during games and training.21, 22, 23 Moreover, the beneficial impact of regular participation in wheelchair rugby training on functional abilities is well documented.24 Although according to general guidelines about the energy expenditure to maintain cardiovascular health and fitness, SCI subjects can ensure the amount of energy expenditure through activities like wheelchair rugby25 and can improve pulmonary function after 1 year of wheelchair rugby training,26 it is not known whether these positive effects also come along with neurophysiological alterations like an increase in BDNF serum concentration.
Thus, the main objectives of this study were (i) to evaluate the influence of a wheelchair rugby training session on serum BDNF concentrations in tetraplegic athletes and (ii) to compare these results with previously reported BDNF concentrations in paraplegic athletes as well as able-bodied subjects at rest and after exercise.
Materials and Methods
Subjects
Eleven male elite wheelchair rugby athletes (age: 31.7±5.9 years; height: 185±0.1 cm; weight: 74±7 kg) with a tetraplegical SCI (spinal lesion level C5-C7; AIS A and B according to the American Spinal Injury Association) were recruited for the study. They were active in wheelchair rugby for 5.7±4.1 years and all members of the German or Polish national team. All subjects were medically checked before the investigation and were informed about the aim, risks and procedures of the tests. All procedures received institutional ethics approval (according to the Helsinki Declaration) and each participant provided written informed consent.
Training session
The training session included a warm-up period, a main section and finally a cool-down. During the warm-up period, the athletes did continuous pushing for about 10 min, followed by eight 20-m submaximal increasing sprints and agility drills. Stretching of the upper extremities completed the warm-up.
The main training section was separated in two segments and contained ball handling, passing drills, scrimmage activity and tactical practice, which lasted 45 min. Parts of a full game were simulated afterwards (second segment). At the end of the training session, the athletes did moderate continuous pushing for cool-down. Overall, the training session lasted 90 min.
At rest, immediately after the warm-up and the first main-training segment (at the same time as the BDNF measurement), heart rate (HR) was measured (FT4, Polar Electro GmbH, Büttelborn, Germany). In addition, the maximum HR during the warm-up and during the first main-training segment was recorded. Capillary blood samples for lactate determination were taken from the earlobe at the same time as HR was measured. For the analysis of lactate concentration, the enzymatic-amperometric measuring method was applied (Biosen C line, EKF, Barleben, Germany).
BDNF measurement
Venous blood samples were taken at rest (after 10 min of sitting in the wheelchair without pushing), immediately (within 1 min) after the warm-up period and after the first half of the main training segment (before the simulated game). These time ranges are within the literature, whereas it has been shown that BDNF values return to baseline after 10–60 min.2 Serum was pipetted after 30 min of sample resting and a following centrifugation at 4000 r.p.m. for 10 min at 4 °C (EBA 21, Hettich, Tuttlingen, Germany). Samples were stored in Eppendorf tubes (Eppendorf, Hamburg, Germany) first for 4 h at −18 °C in a mobile freezer for transport and after that at −40 °C. Enzyme immunoassay ELISA kits (R&D Systems, Minneapolis, MN, USA) were used with an assay range of 62.5–4.000 pg ml−1 and cross-reactivity with one or more available related molecules. Measuring sensitivity was 20 pg ml−1 and intra- and inter-assay variations were 5% and 11%, respectively. The samples were passively defrosted 2 h before analysis and vortexed to ensure homogeneous solutions. The analytical procedure was arranged accurately according to instructions of the kit. The photometric analysis was conducted using a Multiskan FC photometer (Thermo Fisher Scientific Inc., Waltham, MA, USA).
Statistics
All data are shown as mean±s.d. Analysis of variance for repeated measurement was conducted by using the software Statistica (STATSOFT, Tulsa, OK, USA). The rectangular distribution is conducted automatically initially with the analysis of variance by this program. Post hoc comparison with baseline of the significant results were evaluated with the Bonferroni post hoc test. The level of significance for all analyses was set at P⩽0.05.
Results
Heart rate and lactate levels
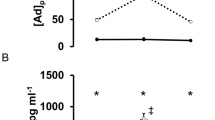
Lactate concentration and HR at rest before exercise differed significantly from values after the warm-up and the main training section (Figure 1).
After the warm-up period, the lactate concentration increased significantly in mean about 316% (P<0.01) and after the first main-training part about 219% (P=0.04) in comparison with rest.
HR increased by 136% (P<0.01) and 144% (P<0.01) in contrast to the rested state. Here, the maximal HR was found during the warm-up (129±16.3 b.p.m.).
BDNF concentration
Before exercise, BDNF concentrations were 33.23±21.58 ng ml−1, after warm up 31.89±18.93 ng ml−1 and during the training session 29.9±10.98 ng ml−1. Analysis of variance revealed no significant (P>0.05) difference between the different measurements.
Discussion
The primary purposes of this study were (i) to investigate the influence of a wheelchair rugby training session on serum BDNF concentrations in tetraplegic athletes and (ii) to compare the results with previously reported values in paraplegics and healthy subjects at rest and after exercise.
In our study, there was no influence of wheelchair rugby training on serum BDNF concentrations. An influence of the increased autonomic dysfunction in tetraplegics on BDNF in comparison with paraplegic subjects could not been shown.
Under the present test conditions, BDNF values of tetraplegic athletes at rest were slightly higher or rather at the upper limit in comparison with previously reported values in able-bodied subjects.2, 3, 27 Knaepen et al.2 summarized a high variability in basal values of 1.5–30.9 ng ml−1 in able-bodied subjects. The data by Rojas Vega et al.20 in paraplegic athletes were somewhat higher than those in our study (Table 1). Increased BDNF concentrations after moderate exercise described in paraplegic athletes20 were not found in the present study with tetraplegical athletes.
In healthy able-bodied persons, lactate concentration seems to be a major mediator for BDNF accumulation. High lactate concentrations elicited by high intensity exercise were linked to increased BDNF values. A potential indicator for the effect of lactate was given by Schiffer et al.7 BDNF concentrations increased immediately after sodium lactate infusion (resulting in blood lactate concentration up to 15 mmol l−1), but not 24 or 60 min after injection. These results were supported by Rojas Vega et al.28 who compared the effects of short low and high intensity exercise with an additional sodium bicarbonate infusion. After high intensity exercise, the BDNF concentrations were elevated until 3 min after exercise. Lactate concentrations remained at the same level in both conditions with and without infusion, while the pH, HCO 3− and base excess were influenced because of the bicarbonate infusion.
To date, there is only one study that examined the influence of exercise intensity on BDNF in SCI (paraplegic) athletes. Rojas Vega et al.20 compared BDNF values at rest, during warm-up and after a marathon simulation on a treadmill. The warm-up intensity was about 54% of HR max and a metabolic situation represented by lactate concentrations of 2.1±1.0 mmol l−1. During a following marathon race simulation, the intensity was about 89% of HR max and an accompanying lactate concentration of 7.5±3.7 mmol l−1. Post-marathon exercise BDNF values were not significantly different compared with resting values before exercise. According to Leicht et al.29 the current metabolic conditions of our subjects already during the warm-up (3.38±1.86 mmol l−1) period but as well at the following main part (2.34±0.92 mmol l−1) of the training session can be estimated as very intensive, whereas BDNF values were not elevated. The influence of high intensity exercise on BDNF levels in able-bodied subjects could not be proved in paraplegic athletes20 nor in the current study in tetraplegic wheelchair rugby athletes. Moreover, there is strong evidence that low-to-moderate intensity exercise leads to raised BDNF levels in SCI athletes and during neurodegenerative diseases.18
The complete and incomplete lesion (AIS A and B) of the current subjects influence physiological factors and can be a parameter of altered neurophysiological reactions in comparison with able-bodied healthy subjects.28 Interestingly, studies30 of BDNF in SCI and multiple sclerosis subjects showed greater standard deviations. Types of lesions, process of diseases like multiple sclerosis may result in greater differences in BDNF concentrations in the rested state.
In healthy able-bodied persons, elevated BDNF levels characterize chronic adaptations through regular physical exercise on a neurophysiological level.2 These results are not validated2 to date and cannot be compared with SCI athletes, while there are no studies comparing trained and untrained subjects.
Conclusion
A typical wheelchair rugby training session has no influence on serum BDNF concentration in tetraplegical SCI athletes. In comparison with previously reported BDNF concentrations in healthy able-bodied subjects at rest, current values were slightly higher or rather at the upper limit in tetraplegic SCI athletes. These findings are in line with BDNF concentrations of paraplegic SCI athletes although the metabolic, respiratory and neurologic situation of these populations differs. In addition, it appears as if the effect of lactate in paralyzed athletes is not comparable with that of able-bodied persons. Intensity and duration of exercise in the present study correspond to a typical training intervention in wheelchair rugby. Further studies should focus on low intensity activities and their impact on neurotrophic factors in SCI.
Data Archiving
There were no data to deposit.
References
Garber CE, Blissmer B, Deschenes MR, Franklin Ba, Lamonte MJ, Lee I-M et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011; 43: 1334–1359.
Knaepen K, Goekint M, Heyman EM, Meeusen R . Neuroplasticity - exercise-induced response of peripheral brain-derived neurotrophic factor: a systematic review of experimental studies in human subjects. Sports Med 2010; 40: 765–801.
Zoladz JA, Pilc A . The effect of physical activity on the brain derived neurotrophic factor: from animal to human studies. J Physiol Pharmacol 2010; 61: 533–541.
Schiffer T, Schulte S, Hollmann W, Bloch W, Strüder HK . Effects of strength and endurance training on brain-derived neurotrophic factor and insulin-like growth factor 1 in humans. Horm Metab Res 2009; 41: 250–254.
Zoladz JA, Pilc A, Majerczak J, Grandys M, Zapart-Bukowska J, Duda K . Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. J Physiol Pharmacol 2008; 59: 119–132.
Ferris LT, Williams JS, Shen C-L . The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med Sci Sports Exerc 2007; 39: 728–734.
Schiffer T, Schulte S, Sperlich B, Achtzehn S, Fricke H, Strüder HK . Lactate infusion at rest increases BDNF blood concentration in humans. Neurosci Lett 2011; 488: 234–237.
De Leon RD, See PA, Chow CH . Differential effects of low versus high amounts of weight supported treadmill training in spinally transected rats. J Neurotrauma 2011; 28: 1021–1033.
Hutchinson KJ, Gómez-Pinilla F, Crowe MJ, Ying Z, Basso DM . Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain 2004; 127: 1403–1414.
Boyce V, Park J . Differential effects of brain‐derived neurotrophic factor and neurotrophin‐3 on hindlimb function in paraplegic rats. Eur J Neurosci 2012; 35: 221–232.
Kim D, Gutin P, Noble L, Nathan D . Treatment with genetically engineered fibroblasts producing NGF or BDNF can accelarte recovery from traumatic spinal cord injury in the adult rat. Neuroreport 1996; 7: 2221–2225.
Ikeda O, Murakami M, Ino H . Acute up-regulation of brain-derived neurotrophic factor expression resulting from experimentally induced injury in the rat spinal cord. Acta Neuropathol 2001; 102: 239–245.
Coumans J V, Lin TT, Dai HN, MacArthur L, McAtee M, Nash C et al. Axonal regeneration and functional recovery after complete spinal cord transection in rats by delayed treatment with transplants and neurotrophins. J Neurosci 2001; 21: 9334–9344.
Seifert T, Brassard P, Wissenberg M, Rasmussen P, Nordby P, Stallknecht B et al. Endurance training enhances BDNF release from the human brain. Am J Physiol Regul Integr Comp Physiol 2010; 298: R372–R377.
Gustafsson G, Lira CM, Johansson J, Wisén A, Wohlfart B, Ekman R et al. The acute response of plasma brain-derived neurotrophic factor as a result of exercise in major depressive disorder. Psychiatry Res 2009; 169: 244–248.
Cui H, Jin Y, Wang J, Weng X, Li C . Serum brain-derived neurotrophic factor (BDNF) levels in schizophrenia: A systematic review. Shanghai Arch Psychiatry 2012; 24: 250–261.
Diniz BS, Teixeira AL . Brain-derived neurotrophic factor and Alzheimer’s disease: physiopathology and beyond. Neuromolecular Med 2011; 13: 217–222.
Gold SM, Schulz K-H, Hartmann S, Mladek M, Lang UE, Hellweg R et al. Basal serum levels and reactivity of nerve growth factor and brain-derived neurotrophic factor to standardized acute exercise in multiple sclerosis and controls. J Neuroimmunol 2003; 138: 99–105.
Schmid A, Huonker M, Barturen J, Stahl F, Ko D, Lehmann M et al. Catecholamines, heart rate, and oxygen uptake during exercise in persons with spinal cord injury. J Appl Physiol (1985) 1998; 85: 635–641.
Rojas Vega S, Abel T, Lindschulten R, Hollmann W, Bloch W, Strüder HK . Impact of exercise on neuroplasticity-related proteins in spinal cord injured humans. Neuroscience 2008; 153: 1064–1070.
Barfield JP, Malone LA, Arbo C, Jung AP . Exercise intensity during wheelchair rugby training. J Sports Sci 2010; 28: 389–398.
Goosey-Tolfrey V, Castle P, Webborn N, Abel T . Aerobic capacity and peak power output of elite quadriplegic games players. Br J Sports Med 2006; 40: 684–687.
Goosey-Tolfrey VL, Leicht CA . Field-based physiological testing of wheelchair athletes. Sports Med 2013; 43: 77–91.
Furmaniuk L, Cywińska-Wasilewska G, Kaczmarek D . Influence of long-term wheelchair rugby training on the functional abilities in persons with tetraplegia over a two-year post-spinal cord injury. J Rehabil Med 2010; 42: 688–690.
Abel T, Platen P, Rojas Vega S, Schneider S, Strüder HK . Energy expenditure in ball games for wheelchair users. Spinal Cord 2008; 46: 785–790.
Moreno MA, Paris JV, Sarro KJ, Lodovico A, Silvatti AP, Barros RML . Wheelchair rugby improves pulmonary function in people with tetraplegia after 1 year of training. J Strength Cond Res 2013; 27: 50–56.
Goda A, Ohgi S, Kinpara K, Shigemori K, Fukuda K, Schneider EB . Changes in serum BDNF levels associated with moderate-intensity exercise in healthy young Japanese men. Springerplus 2013; 2: 678.
Rojas Vega S, Hollmann W, Vera Wahrmann B, Strüder HK . pH buffering does not influence BDNF responses to exercise. Int J Sports Med 2012; 33: 8–12.
Leicht CA, Bishop NC, Goosey-Tolfrey VL . Submaximal exercise responses in tetraplegic, paraplegic and non spinal cord injured elite wheelchair athletes. Scand J Med Sci Sports 2012; 22: 729–736.
Castellano V, White LJ . Serum brain-derived neurotrophic factor response to aerobic exercise in multiple sclerosis. J Neurol Sci 2008; 269: 85–91.
Acknowledgements
We thank Dr Hans Herbert Vater for his support and Gerhard Herrera for providing language help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Zeller, S., Abel, T., Rojas-Vega, S. et al. Brain-derived neurotrophic factor concentrations in tetraplegic athletes. Spinal Cord 53, 791–794 (2015). https://doi.org/10.1038/sc.2015.94
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2015.94