Abstract
Study design:
Experimental study.
Objective:
To investigate the expression of C5a and its receptor after spinal cord ischemia reperfusion injury (SIRI) in rat.
Setting:
Department of Neurosurgery, the Second Affiliated Hospital, Xi’an Jiaotong University, Shaanxi Province, Xi’an, China
Methods:
Sprague Dawley rats were subjected to 1 h of infrarenal aorta occlusion to induce spinal cord ischemia. Spinal cord was reperfused for 12, 24, 48 h or 3 days separately after ischemia, respectively. Enzyme-linked immunosorbent assay was used to detect C5a in rat serum. Immunofluorescent staining was performed to detect the expression and cellular localization of C5aR in spinal cord following SIRI.
Results:
Following SIRI, the motor behavior of the rats was significantly compromised. The serum concentration of C5a in the rat was elevated after SIRI and peaked at 24 h. C5aR was significantly upregulated in the lumbar spinal cord following SIRI and expressed on motor neurons and the microglia, but not astrocyte. There was no significant difference in the expression level of C5aR localized on motor neurons after SIRI. Remarkable upregulation of the C5aR may be associated with increased number of C5aR-positive microglia and elevated cellular expression level.
Conclusion:
This study provides the first evidence of the expression of C5a and its receptors in SIRI and suggests their possible contribution to SIRI in a rat model.
Similar content being viewed by others
Introduction
Spinal cord ischemia reperfusion injury (SIRI) is one of the most devastating complications encountered in many pathological situations, such as surgical procedures on thoracoabdominal aneurysms or the spine. SIRI is mainly contributed by the temporary or permanent ischemia of the spinal cord due to interruption of the blood supply.1 Although various interventions have been reported to prevent or retard SIRI, which still remain unpredictable and unpreventable, current studies suggest that the pathophysiological mechanisms underlying SIRI are complicated, including calcium overload, oxidative stress, excitotoxicity, neuronal apoptosis and the release of inflammatory factor.2, 3 A series of studies3, 4 have revealed that inflammatory activation has a critical role in the progression of SIRI, which include glial response and leukocyte infiltration in the spinal cord. Furthermore, cytokines such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1, IL-6 and IL-10 are all rapidly produced in the spinal cord following SIRI.4
The complement system, a major component of the innate immune system, was constituted by a series of complement components and involved in the neural development, synapse elimination and maturation of neural networks.5 In addition, in a range of brain or spinal cord pathogenesis, including ischemia reperfusion injury (IRI) or stroke, traumatic brain injury and spinal cord injury, rapid disruption of neuronal homeostasis potently triggers complement activation.6 It then produces large amounts of anaphylatoxins C5a, which regulates the immune activation and takes part in other pathophysiological processes via binding with C5a receptor (C5aR) specifically.
Recent studies have shown that the C5a-C5aR signaling pathway was activated in a few central nervous system (CNS) diseases, including CNS inflammation,7 neurodegeneration8, 9 and acute CNS injuries.10, 11, 12 Clinical and experimental studies have also discovered that C5a had an important role in IRI of many organs, including gut, kidney, limb and liver.13 In the spinal cord, C5aR has been demonstrated to be expressed by neurons and glia cells.9 However, little is known about the involvement of C5a and its receptors during the pathogenesis after an ischemic insult of the spinal cord. In the present study, we investigated the spatial-temporal expression profile of C5a and its receptor CD88 following SIRI in the rats, which will be of great benefit for our understanding the mechanism of the complement involvement in SIRI.
Materials and Methods
Experimental protocol
A total of 54 male Spraque Dawley rats weighing 250–300 g were obtained from the Animal Center of Xi’an Jiaotong University, China. The animals were randomly divided into the sham-operated group (sham group, n=9), ischemia group (I group, n=9) and ischemia reperfusion group (IR group, n=36). According the time of reperfusion, the IR group was divided into the IR12h group (n=9), IR24h group (n=9), IR48h group (n=9) and IR3d group (n=9). The SIRI model was established according to Zivin’s process.2 Rats were anesthetized with 0.75% amobarbital sodium (4 ml kg−1) before surgery. A 2.0-cm-long midline abdominal incision was made under aseptic conditions and ischemia was induced by clamping a nontraumatic vascular clip on the abdominal aorta ~0.5 cm below the left renal pedicle for 1 h. After removal of the clamp, the blood supply of spinal cord was restored. The wound was closed and 0.25% bupivacaine was applied topically for postoperative pain management. The neural function of the posterior limbs was evaluated using the Basso, Beattie and Bresnahan (BBB) score. Hematoxylin and eosin (H&E) staining was used to confirm the successful establishment of an animal model. Animals with a satisfied BBB score were killed at each time point indicated before and after reperfusion. Plasma and the L4-L5 spinal cord were collected for future analysis.
Enzyme-linked immunosorbent assay for C5a in plasma
Plasma C5a was detected using an ELISA kit for C5a (Dakewei company, Beijing, China) according to the manufacturer’s specifications. Results are expressed as nanogram per milliliter (ng ml−1) of plasma protein.
Immunofluorescent staining for C5aR protein expression
L4-L5 spinal cords embedded in optimum cutting temperature compound (OCT) were sectioned at 10 μm. Fluorescent double labeling was performed to localize the expression of C5aR on different cell types. Antibodies involved in this study are as follows: mouse anti-rat MAP-2 (1:500 dilution, Abcam, Cambridge, MA, USA); mouse anti-rat GFAP (1:500 dilution, Abcam); mouse anti-rat Iba1 (1:500 dilution, Abcam); rabbit anti-rat C5aR antibody (1:100 dilution, Santa Cruz Biotechnology Inc., Dallas, TX, USA). Sections were blocked (10% goat serum in phosphate-buffered saline (PBS)) for 30 min and then incubated overnight at 4 °C with appropriate primary antibodies. These sections were then washed with PBS and incubated with an appropriate alexa conjugated secondary antibodies: Alexa 488 goat anti-rabbit (1:100 dilution, Santa Cruz Biotechnology Inc.), Alexa 555 goat anti-mouse (1:100 dilution, Santa Cruz Biotechnology Inc.) for 1 h at room temperature. Following PBS rinsing, the sections were incubated for 5 min with 4, 6-diamidino-2-phenylindole (1:15 000, Santa Cruz Biotechnology Inc.). Immunoglobulin G-negative controls were also included to determine nonspecific staining. All sections were mounted in fluorescence mounting medium (Dako, Carpinteria, CA, USA). Images were captured with a Nikon fluorescence microscope (i90, Nikon instruments (Shanghai) Co. Ltd, Shanghai, China). This experiment was repeated in three rats from each group. Five different vision fields of each section from different groups were analyzed using Image-Pro Plus 6.0 (IPP 6.0, Media Cybernetics, Inc., Rockville, MD, USA) software and the average optical densities (OD) were measured.
Statistical analysis
Data were analyzed with SPSS18.0 software (Chicago, IL, USA). One-way analysis of variance was performed for comparation between groups. Results were described as mean±s.d. Statistical significance was accepted for P<0.05.
All applicable institutional and governmental regulations concerning the ethical use of animals were followed during the course of this research.
Results
In order to confirm the successful establishment of the animal model, H&E staining was performed and revealed significant spinal cord damages in the IR group. There were significantly more normal neurons in the anterior horn of the spinal cord in the sham group, where the multipolar structure of neuron was complete. Axons, dendrites and nucleus of neurons were clear, and the nucleolus was located in the center. However, in the IR group, extensive vacuolation, necrotic changes and pyknotic nuclei of neurons were noticed (data not shown).
Plasma concentration of C5a was elevated following SIRI
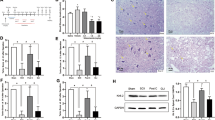
Plasma concentrations of C5a in the IR group were significantly elevated compared with the sham group (Figure 1) (sham group: 1.24±0.32 ng ml−1; IR12h group: 2.16±0.35 ng ml−1, P<0.05; IR24h group: 3.10±0.43 ng ml−1, P<0.05; IR48h group: 2.68±0.38 ng ml−1, P<0.05; IR3d group: 2.44±0.29 ng ml−1, P<0.05, and peaked in the IR24h group. However, the elevation of C5a in the I group did not reach statistical significance. (I group: 1.35±0.32 ng ml−1, P>0.05).
The C5aR expression was upregulated and localized to neurons and microglia, but not astrocytes
Immunofluorescent staining was carried out to detect the expression and cellular localization of C5aR in the lumbar spinal cord. C5aR was mainly expressed on the somas and dendrite of neurons located within the ventral horn of the spinal cord in rats of both the Sham group and IR group. After labeling microglia with Iba1, we found that there were more microglia recruited to injured areas in the spinal cord in the IR group. Furthermore, there was a gradually increased expression of C5aR in Iba1-positive microglia in the IR group (Figure 2a). In addition, we found C5aR present on the somas of neurons (labeled with MAP-2) located within the ventral horn of the spinal cord in the sham group and IR group. However, absence of C5aR was observed on the astrocyte (labeled with GFAP) in the spinal cord (data not shown). The OD values indicated that C5aR expression level was significantly higher in the IR group than Sham group and peaked in the IR24h group (Figure 2b) (P<0.05).
Immunofluorescent staining of C5aR in the spinal cord following SIRI. There was gradually increased expression of the C5aR (green) with strong colocalization with Iba1 (red) in spinal cords of the IR group as time of perfusion prolonged. In comparison, there was little expression of C5aR on microglia in the sham group (a). The OD of C5aR expressions on the lumbar spinal cord of the rats was measured in the three groups and quantified using an image analysis collection system, which showed consistently increased expression levels of C5aR in IR-group rats (b). *P<0.05 vs the sham group. Scale bar, 25 μm; n=9 per group.
Discussion
In the present study, the expression levels of C5a in plasma and its receptor in the spinal cord were increased following SIRI of rats, which indicated that the C5a-C5aR signaling pathway may be activated; however, the downstream pathway would be investigated in the future. Our results add further support to the hypothesis that complement activation may have a role in motor function deficit in SIRI.
The complement system is a pivotal component of the innate immune system, which protects the host from infection and injury. Complement proteins can be induced in all cell types within the CNS, where the signaling pathways seem to have similar roles in host defense. Complement activation induces the cleavage of C5 and produces C5a. C5a is regarded as a potent inflammatory mediator, which recruits and activates immune cells. The primary cellular receptor for C5a (CD88) has been reported to be expressed on all CNS cells, including neurons and glia, indicating functional roles of C5a-C5aR signaling pathway in CNS.7 Therefore, complement activation and the subsequent C5a expression may have a significant role in the progression of CNS disease.6
It has been demonstrated that C5aR expression was greatly upregulated on reactive astrocytes and microglia and to a lesser extent on endothelial cells when inflammation of human CNS was noticed.14 Thus it was proposed that C5aR expression on brain cells has an important role in cell activation and recruitment.7 In acute CNS injury, including traumatic brain injury, ischemic stroke, intracerebral hemorrhage and traumatic spinal cord injury, the upregulation of C5a and/or its receptor was observed in human samples or animal models.10, 11, 12 Furthermore, human and rodent CD88 expression has been found on neurons, glia and endothelial cells within the brain and spinal cord after CNS injury.6 However, expression of C5a and its receptors in response to SIRI has not been reported yet. In the present study, the level of plasma C5a was increased at 12, 24, 48 h and 3 days reperfusion after 1 h ischemia. In addition, the expression of C5aR protein was significantly increased within the spinal cord of rats in the IR group and localized to neurons and activated microglia. Somewhat surprisingly, the C5aR was also found on neurons in the sham group (data not shown). Therefore, it suggested C5a and its receptor had a significant role in the progression of SIRI in a rat model, which may be associated with the increased number of activated microglia and elevated expression of C5aR.
It has been reported that the inhibition or deficiency of C5aR may promote the recovery of histological and neurological function in animals after CNS injuries in recent literature.11, 12 Moreover, inhibiting C5a-C5aR signaling with PMX53 also significantly decreased the infarct volume and improved the neurological function in a mouse cerebral IR model.11 The protective role of C5aR inhibition has also been found in IRI of multiple organs. However, the mechanism remains investigated and probably related to inhibiting inflammation, including attenuation of tissue or serum TNF-α expression, myeloperoxidase activity and the number of infiltrating leukocyte.15, 16, 17, 18, 19 It will be useful to study the underling mechanism of the protective role of inhibiting C5a-C5aR on SIRI for future clinical application.
Data archiving
There were no data to deposit.
References
Lemaire SA, Price MD, Green SY, Zarda S, Coselli JS . Results of open thoracoabdominal aortic aneurysm repai. Ann Cardiothorac Surg 2012; 1: 286–292.
Gong S, Peng L, Yan B, Dong Q, Seng Z, Wang W et al. Bosentan reduces neuronal apoptosis following spinal cord ischemic reperfusion injury. Spinal cord 2014; 3: 181–185.
Gong S, Seng Z, Wang W et al. Bosentan protects the spinal cord from ischemia reperfusion injury in rats through vascular endothelial growth factor receptors. Spinal cord 2014; 2014: 147.
Zhu P, Li J, Fujino M, Zhuang J, Li XK . Development and Treatments of Inflammatory Cells and Cytokines in Spinal Cord Ischemia-Reperfusion Injury. Mediators Inflamm 2013; 2013: 701970.
Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N et al. The classical complement cascade mediates CNS synapse elimination. Cell 2007; 13: 1164–1178.
Woodruff TM, Ager RR, Tenner AJ, Noakes PG, Taylor SM . The role of the complement system and the activation fragment C5a in the central nervous system. Neuromol Med 2010; 12: 179–192.
Gasque P, Singhrao SK, Neal JW, Götze O, Morgan BP . Expression of the receptor for complement C5a (C5aR) is up-regulated on reactive astrocytes, microglia, and endothelial cells in the inflamed human central nervous system. Am J Pathol 1997; 150: 31.
Ager RR, Fonseca MI, Chu SH, Sanderson SD, Taylor SM, Woodruff TM et al. Microglial C5aR (C5aR) expression correlates with amyloid-beta deposition in murine models of Alzheimer's disease. J Neurochem 2010; 113: 389–401.
Woodruff TM, Costantini KJ, Crane JW et al. The complement factor C5a contributes to pathology in a rat model of amyotrophic lateral sclerosis. J Immunol 2008; 181: 8727–8734.
Garrett MC, Otten ML, Starke RM, Komotar RJ, Magotti P, Lambris JD et al. Synergistic neuroprotective effects of C3a and C5a receptor blockade following intracerebral hemorrhage. Brain Res 2009; 1298: 171–177.
Kim GH, Mocco J, Hahn DK, Kellner CP, Komotar RJ, Ducruet AF et al. Protective effect of C5a receptor inhibition after murine reperfused stroke. Neurosurgery 2008; 63: 122.
Li L, Xiong ZY, Qian ZM . Complement C5a is detrimental to histological and functional locomotor recovery after spinal cord injury in mice. Neurobiol Dis 2014; 66: 74–82.
Arumugam TV, Shiels IA, Woodruff TM, Granger DN, Taylor SM . The role of the complement system in ischemia-reperfusion injury. Shock 2004; 21: 401–409.
Van Beek J, Bernaudin M, Petit E, Gasque P, Nouvelot A, MacKenzie ET et al. Expression of receptors for complement anaphylatoxins C3a and C5a following permanent focal cerebral ischemia in the mouse. Exp Neurol 2000; 161: 373–382.
Busche MN, Stahl GL . Role of the complement components c5 and c3a in a mouse model of myocardial ischemia and reperfusion injury. Ger Med Sci 2010; 8: Doc20.
Arumugam TV, Woodruff TM, Stocks SZ, Proctor LM, Pollitt S, Shiels IA et al. Protective effect of a human C5a receptor antagonist against hepatic ischaemia-reperfusion injury in rats. J Hepatol 2004; 40: 934–941.
Arumugam TV, Shiels IA, Strachan AJ, Abbenante G, Fairlie DP, Taylor SM . A small molecule C5a receptor antagonist protects kidneys from ischemia/reperfusion injury in rats. Kidney Int 2003; 63: 134–142.
Woodruff TM, Arumugam TV, Shiels IA, Reid RC, Fairlie DP, Taylor SM . Protective effects of a potent C5a receptor antagonist on experimental acute limb ischemia-reperfusion in rats. J Surg Res 2004; 116: 81–90.
Arumugam TV, Shiels IA, Woodruff TM, Reid RC, Fairlie DP, Taylor SM . Protective effect of a new c5a receptor antagonist against ischemia-reperfusion injury in the rat small intestine. J Surg Res 2002; 103: 260–267.
Acknowledgements
The Natural Science Foundation of China (81300116) contributed to this research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Dong, Q., Sun, L., Peng, L. et al. Expression of C5a and its receptor following spinal cord ischemia reperfusion injury in the rat. Spinal Cord 53, 581–584 (2015). https://doi.org/10.1038/sc.2015.65
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2015.65
This article is cited by
-
Studies on protection against ischemia reperfusion injury after SCI
Spinal Cord (2016)