Abstract
Study design:
A cross-sectional study.
Objectives:
Neuropathic pain (NP) after spinal cord injury (SCI) tends to be hard to treat, and its heterogeneous properties make it difficult to identify and characterize. This study was conducted to assess the characteristics of SCI-related NP in detail.
Setting:
A single hospital for SCI rehabilitation.
Methods:
This study included 72 patients who were seen at our hospital in 2012 and 2013 and who had sustained SCI at least 3 months before enrollment. The patients completed the Neuropathic Pain Symptom Inventory (NPSI) and the Short Form (SF)-36 Health Inventory. The NPSI score was analyzed for correlations with clinical presentations of SCI and SF-36 subitems.
Results:
Paresthesia/dysesthesia was the most common subtype of NP after SCI. With regard to location, below-level superficial NP was significantly more intense than at-level pain. Patients who underwent surgery showed significantly less evoked pain compared with patients with non-surgery. Patients reported significantly more severe pain if >1 year had elapsed after the SCI. Patients with an American Spinal Injury Association Impairment Scale grade of B for completeness of injury reported more intense NP than those with other grades. Among the SF-36 subitems, NP correlated significantly with bodily pain, general health and mental health.
Conclusion:
NP in SCI patients was significantly associated with the location of pain, the time period since the injury, surgery and quality-of-life factors. A more detailed understanding of the characteristics of NP may contribute to better strategies for relieving the pain associated with SCI.
Similar content being viewed by others
Introduction
Pain is a persistent problem for many spinal cord injury (SCI) patients; 65–85% of SCI patients suffer from pain, and one-third of these characterize the pain as severe.1 The pain tends to persist and even worsen with time, especially if it begins within 6 months after the injury.1, 2 Pain takes a toll on the patients’ activity levels and mental health status, reducing their quality of life (QOL).3
A classification system proposed by the International Association of the Study of Pain divides SCI pain into nociceptive and neuropathic pain.4 Nociceptive pain, which arises from the stimulation of peripheral nerves, is common and easily identified, as it increases with movement. However, neuropathic pain, which is defined as pain caused by a lesion or disease of the somatosensory nervous system,4 is harder to identify and characterize because it presents in a variety of ways. There are few specific tools for evaluating neuropathic pain in patients with SCI. Many studies have assessed SCI-related pain using the Visual Analog Scale or a basic Numeric Scale;5 these scales evaluate pain intensity but do not assess subtypes of neuropathic pain. Some studies have used the McGill Pain Questionnaire,6 which is not specific enough to evaluate the neuropathic pain in SCI patients. Other studies have used the Leeds Assessment of Neuropathic Symptoms and Signs7, 8 or the Neuropathic Pain Questionnaire,9 which were designed to diagnose rather than to characterize or evaluate neuropathic pain.
The Neuropathic Pain Symptom Inventory (NPSI) self-questionnaire was specifically designed to evaluate the different symptoms of neuropathic pain in both the peripheral and the central nervous systems,10 and it has been validated for inter-user reproducibility and for sensitivity to change.10 The NPSI divides the characteristics of neuropathic pain into five subgroups: burning (superficial) spontaneous pain, pressing (deep) spontaneous pain, paroxysmal pain, evoked pain and paresthesia/dysesthesia. An 11-point severity rating scale (from 0 to 10) is shown under each question. The words used as descriptors were linguistically validated to ensure that they are both simple and sufficiently specific. These qualities make the NPSI useful for specifying and quantifying subjective pain in daily clinical practice. The NPSI has been validated in reports assessing neuropathic pain after herpes,11 carpal tunnel syndrome12 and spinal cord tumor.13 Its application to neuropathic pain after SCI has not been reported, although a recent study strongly recommended using the NPSI to evaluate this pain.14
The present study was conducted to characterize neuropathic pain in SCI patients using the NPSI scoring system and to clarify the relationship between neuropathic pain and clinical presentation, including QOL, after SCI.
Materials and methods
This study included 72 patients (66 males and 6 females; mean age 56.00 years) who had sustained a SCI at least 3 months before enrollment in the study. These patients were treated at our hospital in 2012 or 2013, as inpatients or outpatients, and were at least 18 years of age when seen.
Neuropathic pain was classified as at-level, below-level or other pain according to the recent updated SCI pain classification;4, 15 briefly, at-level pain was defined as a dermatomal or segmental distribution at the level of injury. Leg pain in patients with an injury at the cauda equina level was classified as at-level neuropathic pain. Below-level pain was defined as a more diffuse distribution below the level of neurological injury. Other pain refers to neuropathic pain that is located above, at or below the lesion site but is not directly related to the SCI.
Completeness of injury was graded (A−D) using the American Spinal Injury Association Impairment Scale (AIS),16 and the level of injury was defined as the lowest level with intact neurological function. Each patient was examined independently by two spine surgeons. Any disagreement between the surgeons was resolved by having both surgeons re-examine the patient together and through discussion.
Each patient completed the NPSI10 and the Short Form (SF)-36 Health Survey after agreeing to participate in the study. Both questionnaires were written in Japanese, and the Japanese-language versions were validated previously.13, 17 We investigated associations between NPSI scores and the injury level, the location of neuropathic pain (at- or below-level), the AIS grade for completeness of injury, the time since the injury and SF-36 scores.
This study was approved by our facility’s Institutional Review Board, and all patients gave informed consent before enrollment.
Statistical analysis
Data were expressed as the mean±s.d. Comparisons of NPSI subscores were assessed by the Kruskal–Wallis and Steel–Dwass tests. Comparisons of NPSI scores with the injury level and AIS grade were assessed by the Kruskal–Wallis test. The Mann–Whitney U-test was used to compare NPSI scores with gender, neuropathic pain location, surgery and length of time since injury. Correlations of NPSI scores with age and SF-36 subitems were assessed by nonparametric Spearman’s test. A P-value <0.05 was considered statistically significant.
Statement of ethics
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
Results
The demographic data and clinical characteristics of the patients are summarized in Table 1. The mean time between the injury and the examination at enrollment was 73 months (range, 3–525 months). Sixty-three out of 72 patients (87.50%) reported neuropathic pain (Table 1).
The mean NPSI score was 13.01±11.68 out of 50 possible points; 38 patients (52.78%) scored 10 or less (mild pain), and 34 patients (47.22%) had a score >10 (moderate-to-severe pain). There were no significant correlations between the NPSI score and age (P=0.575), injury level (P=0.812) or gender (P=0.476).
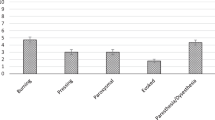
Detailed analysis of the NPSI subscores showed significantly higher scores for paresthesia/dysesthesia (3.80±2.97) than for burning spontaneous pain (2.46±3.21), pressing spontaneous pain (2.27±2.69), paroxysmal pain (2.07±2.78) or evoked pain (2.51±2.71; Figure 1); there were no other statistically significant differences between any two subscores. We also examined correlations by injury level. At the cervical level, the scores for paresthesia/dysesthesia (3.98±2.90) were significantly higher than those for burning spontaneous pain (2.50±3.24), pressing spontaneous pain (2.27±2.56) or paroxysmal pain (2.04±2.89; Figure 1). There were no significant differences between subscores at the thoracic (P=0.713) and lumbar levels (P=0.188).
Total NPSI subscores and NPSI subscores according to the injury level (cervical, thoracic or lumbar). For all injury levels, the paresthesia/dysesthesia scores were significantly higher than those for other pain subtypes. The scores for paresthesia/dysesthesia were also significantly higher than the other subscores for cervical-level injuries. * P<0.05; ** P<0.01.
To clarify the relationship between the NPSI score and the location of neuropathic pain, we compared patients with only at-level pain (n=28) or below-level pain (n=11); patients with both at- and below-level pain or without pain were excluded (Table 1; Figure 2). There was no patient classified to have other neuropathic pain. There was a trend toward a higher total NPSI score in patients with below-level pain (21.30±13.27) rather than at-level pain (12.68±9.18; P=0.0658). A significantly higher subscore for burning spontaneous pain was observed in patients with below-level pain (4.70±3.27) compared with those with at-level pain (2.18±2.96; P=0.0227). There were no other significant differences in the subscores of the two groups (pressing spontaneous pain: P=0.705; paroxysmal pain: P=0.094; evoked pain: P=0.302; paresthesia/dysesthesia: P=0.0965).
We also analyzed the NPSI scores according to the AIS grade for completeness of injury (Figure 3) and found that there was a trend toward higher NPSI scores in patients with AIS grade B (28.50±13.91) compared with those with other grades (A: 10.76±10.43; C: 11.81±10.23; D: 13.50±12.10; P=0.0649).
With respect to the association of the NPSI score with surgery (Figure 4), the patients who underwent surgery showed a lower NPSI score (10.87±10.60) compared with the patients without surgery (16.30±12.67), although there was no significant difference (P=0.0775). When examining the NPSI subscores, however, the patients with surgical treatment showed a significantly lower score in evoked pain (1.84±2.18) than the ones with non-surgery (3.44±3.19; P=0.0422). In the other subscores, there were no significant difference between the groups in burning spontaneous pain (P=0.261), pressing spontaneous pain (P=0.161), paroxysmal pain (P=0.0884) and paresthesia/dysesthesia (P=0.125).
Regarding the time after SCI, patients assessed >1 year after SCI (n=37) had significantly higher NPSI scores than those assessed within a year (n=35) (26.66±24.72 vs 13.27±13.04; P=0.0027; Figure 5). In addition, a lower proportion of patients responding >1 year after SCI had at-level pain, and a higher proportion had below-level pain, compared with the patients who were assessed within a year of SCI (Table 2). These results suggest that patients suffer more intense below-level neuropathic pain over time (Figures 2 and 5).
For the 63 patients who experienced neuropathic pain, the analysis for correlations between the NPSI score and individual SF-36 subitem scores found moderate but significant negative correlations between the NPSI score and bodily pain, general health and mental health (Table 3), but no significant correlations between the NPSI score and the physical function, physical role, vitality, social function or emotional role.
Discussion
This study was conducted to clarify the relationships between neuropathic pain and clinical presentation, including QOL, after SCI. Using the NPSI scoring system, we found that paresthesia/dysesthesia was the most common neuropathic pain-related complaint after SCI. Neuropathic pain was significantly associated with the location of pain, duration after injury and QOL factors. The simple, validated NPSI self-questionnaire was effective for assessing the characteristics of SCI-related neuropathic pain in detail.
Of the five NPSI subtypes of neuropathic pain, paresthesia/dysesthesia was the most commonly reported by patients with SCI. This agrees with a previous report that the prevalence of dysesthesia after SCI is ~80%.18 Likewise, studies using the NPSI questionnaire report that paresthesia/dysesthesia is the most frequent and intense type of neuropathic pain in patients with carpal tunnel syndrome12 and postoperative intramedullary spinal cord tumor.13 In contrast, the predominant type of neuropathic pain associated with herpes zoster is burning spontaneous pain.11 These findings indicate that the NPSI is effective for clarifying and distinguishing the differing characteristics of neuropathic pain in various diseases and pathological conditions.
We found that, although statistical significance was not reached, below-level neuropathic pain was more intense than at-level pain in the total NPSI scores. Similarly, a prospective study by Siddall et al.19 showed that, 6 months after SCI, patients with below-level neuropathic pain were more likely to report their pain as severe or excruciating than those with at-level pain. When examining the breakdowns of pain characteristics in our study, burning spontaneous pain was significantly more intense in the patients with below-level pain than in those with at-level pain. Below-level pain is believed to reflect severe damage and degeneration of the spinothalamic tract.13 Therefore, our results indicate that neuropathic pain originating from the damaged spinothalamic tract might be experienced mainly as superficial spontaneous pain by SCI patients.
Our study found that patients with AIS grade B showed a trend toward more intense neuropathic pain than patients with other AIS grades. Although a relationship between completeness of injury and prevalence of pain was previously reported,1, 20 ours is the first study to clarify the association of completeness of injury with the intensity of neuropathic pain. Analysis of the proportion of patients with below-level pain in each grade showed that patients with grade B had the highest frequency of below-level pain (80%), reflecting the most intense pain in this group.
The reason why patients with AIS grade B had the greatest pain remains unclear. Past reports do not offer much insight, because very few have examined the relationship between SCI grading and pain. However, Siddall et al.19 similarly reported that the prevalence of allodynia was significantly higher in patients with incomplete SCI than in those with complete SCI. A plausible explanation is that, although the neural pathways in cases of complete SCI are interrupted at the injury site, there are spared tracts in cases of incomplete SCI that enable the conduction of pain sensation across the lesion.21 Perhaps the ratio of injured to spared tracts is what determines the intensity of neuropathic pain, resulting in the greatest neuropathic pain in grade B patients, whereas patients with AIS grades C and D have more sensation from spared tracts.
A previous study examined the association of neuropathic pain with surgery and concluded that there were no significant differences in the prevalence of neuropathic pain in surgical and non-surgical groups.22 However, this study just conducted qualitative analysis to examine whether the neuropathic pain was present or absent in SCI patients. In contrast, our quantitative analysis for SCI neuropathic pain revealed that the patients who underwent surgery showed significantly less severe evoked pain compared with the ones with non-surgery. Although surgical indication and approach were left to the discretion of surgeon in each institute, the results of our study suggest that surgical intervention could contribute to the reduction in neuropathic pain after SCI.
Our results also demonstrated that the patients who completed the NPSI questionnaire >1 year after SCI reported more intense neuropathic pain than those responding within a year of injury. A previous study found that at-level neuropathic pain had an earlier onset than below-level pain.1 Consistent with these findings, our data showed that a higher proportion of patients responding >1 year after SCI had below-level pain than patients responding within a year, suggesting that the later onset of intense below-level pain may have contributed to the higher NPSI score.
Our study found significant correlations between neuropathic pain and SF-36 subscores for bodily pain, general health and mental health; a previous study also found a strong correlation between mood or depression and neuropathic pain.23 However, neuropathic pain did not correlate with any other SF-36 subitems. It is reasonable that neuropathic pain did not correlate with all QOL factors, as SCI patients are affected not only by pain but also by limitations in moving, walking and controlling egestion and sexual function.1 Thus, neuropathic pain is one of the several factors that should be considered and treated comprehensively to improve the QOL in SCI patients.
In our study, neuropathic pain was diagnosed using the International Association of the Study of Pain definitions.4 Other studies have diagnosed neuropathic pain using screening tools such as the Leeds Assessment of Neuropathic Symptoms and Signs or the Neuropathic Pain Questionnaire.7, 8, 9 Although these diagnostic tools vary in reliability and validity,24 their use along with the NPSI scoring system might reveal more precise characteristics of the neuropathic pain in SCI.
Study limitations
This study has several limitations. First, the number of patients was small, which reduced the statistical power. In particular, although the patients with AIS grade B presented with the most severe neuropathic pain, there were only five patients in this grade. As SCI presents with heterogeneous clinical characteristics, further research should be conducted with a larger sample size to validate our results. Second, as this was not a randomized study or consecutive case series, the possibility of selection and information bias cannot be ruled out. Finally, we could not assess the changes in pain in individual patients in this cross-sectional study. A multicenter prospective cohort study using NPSI scores should be performed to obtain a more complete assessment of the neuropathic pain in SCI.
Conclusions
The most common subtype of neuropathic pain after SCI was paresthesia/dysesthesia. Neuropathic pain was significantly associated with the location of pain, duration after injury, surgery and some QOL factors. The NPSI scoring system was effective for characterizing SCI-related neuropathic pain, and this detailed characterization may improve our understanding of and ability to treat neuropathic pain in SCI patients.
Data archiving
There were no data to deposit.
References
Siddall PJ, McClelland JM, Rutkowski SB, Cousins MJ . A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain 2003; 103: 249–257.
Jensen MP, Kuehn CM, Amtmann D, Cardenas DD . Symptom burden in persons with spinal cord injury. Arch Phys Med Rehabil 2007; 88: 638–645.
Rintala DH, Loubser PG, Castro J, Hart KA, Fuhrer MJ . Chronic pain in a community-based sample of men with spinal cord injury: prevalence, severity, and relationship with impairment, disability, handicap, and subjective well-being. Arch Phys Med Rehabil 1998; 79: 604–614.
Bryce TN, Biering-Sorensen F, Finnerup NB, Cardenas DD, Defrin R, Lundeberg T et al. International spinal cord injury pain classification: part I. Background and description. March 6-7, 2009. Spinal Cord 2012; 50: 413–417.
Norrbrink Budh C, Hultling C, Lundeberg T . Quality of sleep in individuals with spinal cord injury: a comparison between patients with and without pain. Spinal Cord 2005; 43: 85–95.
Melzack R . The McGill Pain Questionnaire: major properties and scoring methods. Pain 1975; 1: 277–299.
Bennett M . The LANSS Pain Scale: the Leeds assessment of neuropathic symptoms and signs. Pain 2001; 92: 147–157.
Jang JY, Lee SH, Kim M, Ryu JS . Characteristics of neuropathic pain in patients with spinal cord injury. Ann Rehabil Med 2014; 38: 327–334.
Krause SJ, Backonja MM . Development of a neuropathic pain questionnaire. Clin J Pain 2003; 19: 306–314.
Bouhassira D, Attal N, Fermanian J, Alchaar H, Gautron M, Masquelier E et al. Development and validation of the Neuropathic Pain Symptom Inventory. Pain 2004; 108: 248–257.
Bouhassira D, Chassany O, Gaillat J, Hanslik T, Launay O, Mann C et al. Patient perspective on herpes zoster and its complications: an observational prospective study in patients aged over 50 years in general practice. Pain 2012; 153: 342–349.
Truini A, Padua L, Biasiotta A, Caliandro P, Pazzaglia C, Galeotti F et al. Differential involvement of A-delta and A-beta fibres in neuropathic pain related to carpal tunnel syndrome. Pain 2009; 145: 105–109.
Nakamura M, Tsuji O, Iwanami A, Tsuji T, Ishii K, Toyama Y et al. Central neuropathic pain after surgical resection in patients with spinal intramedullary tumor. J Orthop Sci 2012; 17: 352–357.
Calmels P, Mick G, Perrouin-Verbe B, Ventura M . SOFMER (French Society for Physical Medicine and Rehabilitation). Neuropathic pain in spinal cord injury: identification, classification, evaluation. Ann Phys Rehabil Med 2009; 52: 83–102.
Widerstrom-Noga E, Biering-Sorensen F, Bryce TN, Cardenas DD, Finnerup NB, Jensen MP et al. The International Spinal Cord Injury Pain Basic Data Set (version 2.0). Spinal Cord 2014; 52: 282–286.
Maynard FM Jr., Bracken MB, Creasey G, Ditunno JF Jr., Donovan WH, Ducker TB et al. International Standards for Neurological and Functional Classification of Spinal Cord Injury. American Spinal Injury Association. Spinal Cord 1997; 35: 266–274.
Fukuhara S, Ware JE Jr., Kosinski M, Wada S, Gandek B . Psychometric and clinical tests of validity of the Japanese SF-36 Health Survey. J Clin Epidemiol 1998; 51: 1045–1053.
Finnerup NB, Johannesen IL, Sindrup SH, Bach FW, Jensen TS . Pain and dysesthesia in patients with spinal cord injury: a postal survey. Spinal Cord 2001; 39: 256–262.
Siddall PJ, Taylor DA, McClelland JM, Rutkowski SB, Cousins MJ . Pain report and the relationship of pain to physical factors in the first 6 months following spinal cord injury. Pain 1999; 81: 187–197.
Werhagen L, Budh CN, Hultling C, Molander C . Neuropathic pain after traumatic spinal cord injury—relations to gender, spinal level, completeness, and age at the time of injury. Spinal Cord 2004; 42: 665–673.
Chang Y, Jung TD, Yoo DS, Hyun JK . Diffusion tensor imaging and fiber tractography of patients with cervical spinal cord injury. J Neurotrauma 2010; 27: 2033–2040.
Sved P, Siddall PJ, McClelland J, Cousins MJ . Relationship between surgery and pain following spinal cord injury. Spinal Cord 1997; 35: 526–530.
Ataoglu E, Tiftik T, Kara M, Tunc H, Ersoz M, Akkus S . Effects of chronic pain on quality of life and depression in patients with spinal cord injury. Spinal Cord 2013; 51: 23–26.
Hallstrom H, Norrbrink C . Screening tools for neuropathic pain: can they be of use in individuals with spinal cord injury? Pain 2011; 152: 772–779.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Nagoshi, N., Kaneko, S., Fujiyoshi, K. et al. Characteristics of neuropathic pain and its relationship with quality of life in 72 patients with spinal cord injury. Spinal Cord 54, 656–661 (2016). https://doi.org/10.1038/sc.2015.210
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2015.210
This article is cited by
-
Factors associated with neuropathic pain in Colombian patients with spinal cord injury of traumatic origin: case–control study
Spinal Cord Series and Cases (2022)
-
The association of Type D personality with functional outcomes, quality of life and neuropathic pain in persons with spinal cord injury
Spinal Cord (2022)
-
Neuropathic pain after spinal intradural benign tumor surgery: an underestimated complication?
Neurosurgical Review (2022)
-
Pain characteristics in Italian people with spinal cord injury: a multicentre study
Spinal Cord (2022)
-
Which factors have an association to the Quality of Life (QoL) of people with acquired Spinal Cord Injury (SCI)? A cross-sectional explorative observational study
Spinal Cord (2021)