Abstract
Study design:
Prospective, randomized, controlled parallel group trial with single-blinded data analysis.
Objectives:
To determine the safety and efficacy of higher (20 ml kg−1 ideal body weight (IBW)) vs standard (10 ml kg−1 IBW) tidal volumes (Vt) for patients with sub-acute traumatic tetraplegia during ventilator weaning using a 14-day (minimum) weaning protocol.
Setting:
United States regional spinal cord injury treatment center.
Methods:
Thirty-three ventilator requiring inpatients were randomized to either the higher (Group 1) or the standard (Group 2) Vt protocol. Initially, all patients were ventilated at 10 ml kg−1 IBW Vt and 5 cm H2O of PEEP for 72 h. For Group 1, Vt was raised 100 ml kg−1 until reaching target Vt of 20 ml kg−1 IBW. Group 2 was maintained at Vt of 10 ml kg−1 IBW. Plateau pressures were kept at or below 30 cm H2O. Safety outcomes included incidence of adverse events.
Results:
Because of smaller than expected enrollment, evaluation of efficacy was not possible. Therefore, we report the safety outcomes of 33 study participants. The 16 patients in Group 1 and 17 patients in Group 2 were demographically similar at baseline, except for age. The average age was 39.3 years in Group 1 and 27.2 years in Group 2, (P=0.002). There was no difference in median days to wean: 14.5 days in Group 1 and 14 days in Group 2. The incidence of adverse pulmonary events was similar between groups.
Conclusion:
Higher tidal volumes can be safely utilized during weaning of patients with tetraplegia from mechanical ventilation using a 14-day weaning protocol.
Similar content being viewed by others
Introduction
There are ~12 000 new spinal cord injury (SCI) cases each year in the United States, and life expectancy for those individuals is still significantly below those without SCI.1 Data from the National Spinal Cord Injury Statistical Center in the United States indicate that people with SCI who are ventilator dependent have approximately half the life expectancy of similarly injured non-ventilated individuals, and the cause of death is respiratory in over 50% of cases.1, 2, 3 Reaching ventilator liberation early in the rehabilitation course not only increases life expectancy but also improves quality of life, decreases costs3, 4, 5 and enhances participation in rehabilitation. There is no consensus, however, on the best method of weaning SCI patients or on the optimal tidal volume (Vt) to be used. The Consortium for Spinal Cord Medicine’s 2005 Clinical Practice Guidelines “Respiratory Management Following Spinal Cord Injury”5 recommends the use of high Vt (20–25 ml kg−1 ideal body weight (IBW; formula: males: IBW (kg)=50+2.3 (height (in)–60); females: IBW (kg)=45.5+2.3 (height (in)–60))) in people with SCI, but the safety of higher Vt has been questioned.
The use of higher Vt in a general population at risk for acute respiratory distress syndrome (ARDS) is associated with worse outcomes including organ failure, ARDS, prolonged intensive care unit stays and increased mortality.6, 7 Acute SCI patients are certainly at higher risk for ARDS/acute lung injury and death8 and should be managed with a low tidal volume protocols. Sub-acute SCI patients, however, are generally not felt to be at risk for ARDS and may not need lung-protective ventilation. In fact, they may be more prone to atelectasis and wean failure if a low Vt strategy is used.9 High Vts have been safely used in the sub-acute SCI population for many years and are believed to help prevent atelectasis, minimize pulmonary complications, augment speech and enhance weaning.10, 11 This study addresses the safety of using higher Vt in a pre-designated minimum 14-day protocol of ventilator weaning for sub-acute SCI patients who were not felt to be at risk for ARDS.
Patients and methods
Participants
SCI patients who required mechanical ventilation provided informed consent and were screened for eligibility criteria that included injury within the past 6 months, traumatic SCI at cervical levels C3–C6 and non-functional motor preservation as measured by the American Spinal Injury Association Impairment Scale (AIS) and respiratory failure requiring continuous mechanical ventilation. All subjects underwent diaphragmatic fluoroscopy prior to entry into the study to eliminate individuals who demonstrated paralysis of the diaphragms. The complete eligibility criteria are listed in Table 1.
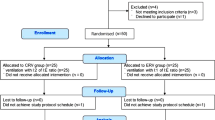
Those who met the criteria for participation were enrolled in the study. We stratified participants into two groups based on neurologic injury level, as the C5–6 subgroup was expected to wean more readily than the C3–4 group. The flow of participants through the study is illustrated in Figure 1.
Intervention
Participants were ventilated in assist control mode at 10 ml kg−1 IBW with 5 cm H2O positive end expiratory pressure (PEEP) for 72 h prior to being randomized into a standard (10 ml kg−1 IBW) or a higher (20 ml kg−1 IBW) Vt group. The use of PEEP was standard of care at the institution and has been shown to be protective of ventilator-induced lung injury if higher Vts are used.12 Plateau pressures (Pplat) were kept ⩽30 cm H2O for both groups. Following the stabilization period, the Vt in Group 1 was titrated upward at a rate of 100 ml day−1 until 20 ml kg−1 IBW was reached. PEEP was maintained at 5 cm H2O for both groups. The wean protocol commenced during the upward Vt titration and consisted of progressive, spontaneous breathing periods (Table 2). Arterial blood gases were monitored weekly. The ventilator dead space was adjusted to maintain a pCO2 of at least 32 to prevent progressive respiratory alkalosis. The Borg Scale13 was obtained after each weaning episode to assess subjective dyspnea.
A participant was maintained at each step of the wean protocol for a minimum of 1 day and advanced to the next step if study criteria were met. Participants were required to repeat a step in the protocol if they failed to meet the prescribed criteria (including but not limited to increased O2 requirements and participant request; Table 3). Our standard of care rehabilitation program of physical and occupational therapy, nursing care, education and psychosocial counseling was maintained during weaning and after ventilator liberation. In addition, participants in both groups received our standard of care for medication management of respiratory signs and symptoms including albuterol, N-acetylcysteine, inhaled budesonide, guaifenesin for secretion clearance and ipratropium as needed.
Outcomes
Outcome measurements included successful completion of the wean protocol, respiratory mechanics, chest x-ray findings and dyspnea as measured by the Borg scale. Each assessment was recorded at baseline and then weekly throughout the study period. All imaging studies were subsequently reviewed to confirm the presence of abnormal findings (atelectasis, infiltrates, edema, ARDS or barotrauma) by a thoracic radiologist and two study pulmonologists who were blinded as to the patient’s treatment arm.
The primary efficacy outcome was to examine days to ventilator liberation (72 h ventilator free). Secondary efficacy outcomes were improvement in specific pulmonary functions chosen for their reproducibility and clinical significance: forced vital capacity (FVC), peak inspiratory pressure (PIP) and Pplat. PIP and Pplat were monitored to assess risk for barotrauma and development of ARDS.
The primary safety outcome was the incidence of key pulmonary adverse events (ventilator-associated pneumonia (VAP), barotrauma and ARDS). VAP was defined as the development of new or progressive pulmonary infiltrates in conjunction with at least two out of three clinical findings of abnormal temperature (>38 °C or <36 °C), leukocytosis or leukopenia (WBC>12 000 or <40 000 or >10% immature forms) and purulent tracheobronchial secretions.14 Barotrauma was defined as pneumothorax, pneumomediastinum, pneumopericardium or pneumatocoele. ARDS was diagnosed using the 1994 American-European Consensus Conference/AECC definition.15 Additional safety end points were development of atelectasis, requirement for secretion clearance maneuvers (suction/cough-assist), measurement of dyspnea and non-pulmonary adverse events. Atelectasis was detected by weekly chest radiograph and confirmed by the reviewing study physicians. Need for secretion clearance maneuvers was documented prospectively with each ventilator assessment and weaning trial by the respiratory therapist. The participant’s perceived dyspnea as measured by the Borg scale was recorded following each wean episode.
Statistical plan
It was originally proposed that survival analysis (Cox regression) would be used to compare the two groups on time to ventilator liberation; however, this analytic approach was abandoned for two reasons: (i) the sample size captured (n=33) rendered the study underpowered (originally, the study was powered based on a sample of 70); and (ii) the imposed weaning schedule resulted in the vast majority participants achieving ventilator liberation in 14 days. Thus, the main focus of the analyses investigated higher Vt safety compared with lower Vt, focusing on comparison of adverse outcomes between the two groups. Descriptive techniques (that is, mininum, maximum and quartiles) were utilized with the purpose of demonstrating that, for both groups, time to wean was nearly identical. Analyses were also performed on secondary outcomes where group comparisons consisted of t-tests for continuous variables, tests for equality of proportions for categorical variables (using Fisher’s exact test) and reporting of odds ratios. P-values associated with these tests should be considered for descriptive purposes and to potentially inform a larger and a sufficiently powered study.
Statement of ethics
We certify that all applicable institutional and governmental regulations concerning the ethical use of human subject volunteers were followed during the course of this research. The study was approved by the HealthONE Alliance Institutional Review Board.
Trial registration
The trial was registered on clinicaltrials.gov (identifier number RCT 00412308).
Results
The study consisted of 33 participants randomly assigned into a higher and a standard Vt group. (Figure 1). The enrollment was stopped short of the target due to fewer than expected admissions of eligible patients during the 4-year study period combined with a higher than expected ineligibility rate. There were no significant differences between the two groups in demographic and injury characteristics including gender, cause of SCI, SCI level or presence/absence of diaphragm paralysis, although three individuals in the higher Vt group had evidence of hemidiaphragm weakness (Table 4). The mean age of the two groups at baseline was statistically different––39.3 years for the higher Vt group and 27.2 years for the standard Vt group (P=0.002). Because of the difference in age between the two groups, age was statistically controlled for by treating it as a covariate in investigation of the primary and secondary outcomes.
Primary outcome
There was no significant difference in the number of days to wean from mechanical ventilation between the two treatment groups. Both groups took a mean of 14 days to achieve >72 h of ventilator liberation. When analyzed by subgroup of SCI level (C3–4 vs C5–6), both subgroups weaned at 14 days, reflecting no difference in median days to ventilator liberation. After controlling for age, there was likewise no difference in median days to wean based on a hazard ratio of 1.163 with an associated 95% confidence interval ranging from 0.485 to 2.790 (P=0.7349).
Secondary outcomes
The FVC values improved over time in both groups, but there was no significant difference in the rate of improvement between groups (P=0.172).
Although the PIP and Pplat values for all subjects remained in a safe range throughout the trial, differences between the groups were observed. For each day of weaning, the higher Vt group had an additional 0.5814 cm H2O increase in mean PIP and an additional 0.4133 cm H2O increase in mean Pplat compared with the standard Vt group (P<0.0001).
Safety outcomes
The odds of developing VAP did not differ between the two Vt groups (odds ratio: standard Vt vs high Vt=1.56, (P=0.607)). The average total number of VAPs reported did not differ between treatment groups (P=0.1597). The higher Vt group reported 4 episodes of VAP and the standard Vt group reported 3 episodes of VAP for a total of 7 VAPs out of 33 patients. There was also no difference between treatment groups when analyzed by SCI level (C3–4 or C5–6) in the odds of developing VAP. The odds of developing a VAP in the standard Vt group was 2.25 (0.5383) in the C3–4 subgroup and 1.25 (0.8530) in the C5–6 subgroup. Neither ARDS nor barotrauma occurred in any study subjects. Borg dyspnea scores and the use of airway clearance maneuvers also did not differ between groups (0.7496).
The number of pulmonary adverse events (VAP, atelectasis, dyspnea and secretions) did not differ between the two groups. The number of ALL adverse events combined within the two groups did not significantly differ from each other. A listing of adverse events appears in Table 5.
Discussion
We did not find a significant difference between the higher Vt and the standard Vt groups for the safety outcomes. Both groups successfully weaned off the ventilator at ~14 days, and there was no increased incidence of VAP, ARDS or barotrauma in the higher Vt group.
In recent years, the safety of higher Vts has been questioned. Studies suggest that higher Vts of 10–15 ml kg−1 IBW may promote ventilator-induced lung injury by producing alveolar over-distention (volutrauma), high airway pressures (barotrauma) and repetitive alveolar expansion and collapse (atelectatrauma).12, 16 In a landmark multi-center, randomized trial published in the New England Journal of Medicine in 2000, the ARDS Network investigators found that the overall mortality was significantly lower, and ventilator-free days were significantly greater when individuals with ARDS were ventilated with low Vt (6 ml kg−1 IBW).7 This study has changed the standard of care for patients with ARDS by mandating the use of a lung-protective ventilator strategy in patients at risk for ARDS. Whether these recommendations apply to other patient populations without identifiable risk factors for ARDS is unclear. Sub-acute SCI patients are generally not felt to be at the same risk for ARDS, as acute SCI patients and higher Vts have been safely used in these patients for many years.10, 11, 17 Current clinical practice guidelines even advocate higher Vts in SCI patients5 because higher Vts have been associated with improved outcomes. A retrospective concurrent cohort comparison study of SCI patients found that higher Vts (mean Vt 25.3 ml kg−1 IBW) were associated with earlier weaning from mechanical ventilation and more rapid resolution of atelectasis than lower Vts (mean Vt 15.5 ml kg−1 IBW).11 A more recent retrospective review also suggested that the use of higher Vts resulted in fewer days on mechanical ventilation.17
Large Vts prevent small-airway closure by stretching airway smooth muscle and reduce surface tension by expanding the surface area of pulmonary surfactant. Tidal volumes as large as 1.0 litre are still only about 1/3 the inspiratory capacity of an average adult and are not felt to cause ventilator-associated lung damage in the absence of acute lung injury from other causes.18 In addition, higher Vts are often preferred by people with SCI because they help augment speech, decrease the work of breathing and the sensation of dyspnea.19 Our study was not able to confirm the superiority of higher Vts in the time to wean, but we did not find any adverse outcomes using this strategy. Specifically, there were no incidences of ARDS or barotrauma in our study, even in the higher Vt group.
The study was limited by its strict adherence to our institutional wean protocol, which prohibited 'skipping' steps, thus requiring a minimum wean duration of 14 days regardless of a participant’s ability to wean in a shorter time frame. For individuals who were not doing well with weaning, our protocol allowed for lengthening the weaning time. However, as there was a clustering of wean duration outcomes at 14 days in both groups, the protocol was insensitive to any real difference in efficacy that might have been detected with a protocol that allowed shorter weaning duration. We chose to have both groups adhere to a protocol because it was previously shown that having a designated protocol allows people with SCI to gradually improve endurance and strength, which leads to better outcomes.20
We did not achieve the anticipated enrollment due to lower than expected admissions of individuals meeting the study criteria, and this decreased the statistical power of the study. This study cannot be considered a definitive trial on the efficacy of one Vt strategy or the other but does provide important information on the duration of weaning and the safety of using higher Vts in patients with sub-acute SCI.
Conclusion
There was no significant difference in the time to wean, incidence of VAP or occurrence of adverse events in patients with tetraplegia who were ventilated with Vt of 10 ml kg−1 IBW vs 20 ml kg−1 IBW. Mechanical ventilation at higher Vts was not associated with any episodes of ARDS or barotrauma in this small randomized trial. Whether higher Vts translate to improved outcomes and acceptable safety in this unique patient population needs to be addressed in a larger randomized controlled trial.
Data archiving
There were no data to deposit.
References
National Spinal Cord Injury Statistical Center. Spinal Cord Injury Facts and Figures at a Glance. University of Alabama at Birmingham: Birmingham, Alabama. 2013.
DeVivo MJ, Krause JS, Lammertse DP . Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil 1999; 80: 1411–1419.
Watt JW, Wiredu E, Silva P, Meehan S . Survival after short- or long-term ventilation after acute spinal cord injury: a single-centre 25-year retrospective study. Spinal Cord 2011; 49: 404–410.
Charlifue S, Apple D, Burns SP, Chen D, Cuthbert JP, Donovan WH et al. Mechanical ventilation, health, and quality of life following spinal cord injury. Arch Phys Med Rehabil 2011; 92: 457–463.
Consortium for Spinal Cord Medicine. Clinical Practice Guideline: respiratory management following spinal cord injury: a clinical practice guideline for health-care professionals. Paralyzed Veterans Am 2005; 28: 259–293.
Lellouche F, Dionne S, Simard S, Bussieres J, Dagenais F . High tidal volumes in mechanically ventilated patients increase organ dysfunction after cardiac surgery. Anesthesiology 2012; 116: 1072–1082.
ARDS Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and acute respiratory distress syndrome. N Engl J Med 2000; 342: 1301–1308.
Veeravagu A, Jiang B, Rincon F, Malenfort M, Ratliff JK . ARDS and ALI in patient with vertebral column fractures and SCI: a nationwide inpatient sample study. Spinal Cord 2013; 51: 461–465.
Wongsurakiat P, Pierson DJ, Rubenfeld GD . Changing pattern of ventilator settings in patients without acute lung injury: changes over 11 years in a single institution. Chest 2004; 126: 1281–1291.
Padman R, Alexander M, Thorogood C, Porth S . Respiratory management of pediatric patients with spinal cord injuries: retrospective review of the duPont experience. Neurorehab Neural Re 2003; 17: 32–36.
Peterson WP, Barbalata L, Brooks CA, Gerhart KA, Mellick DC, Whiteneck GG . The effect of tidal volumes on the time to wean persons with high tetraplegia from ventilators. Spinal Cord 1999; 37: 284–288.
Slutsky AS, Ranieri VM . Ventilator-Induced Lung Injury. N Engl J Med 2013; 369: 2126–2136.
Borg GA . Psychophysical bases of perceived exertion. Med Sci Sport Exer 1982; 14: 377–381.
Dodek P, Keenan S, Cook D, Heyland D, Jacka M, Hand L et al. Evidence-based clinical practice guideline for prevention of ventilator-associated pneumonia. Ann Int Med 2004; 141: 305–313.
Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L et al. The American-European Consensus Conference on ARDS Definitions: mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994; 149: 818–824.
Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA 1999; 282: 54–61.
Wong SL, Shem K, Crew J . Specialized respiratory management for acute cervical spinal cord injury: a restrospective analysis. Top Spinal Cord Inj Rehabil 2012; 18: 283–290.
Brown R, DiMarco AF, Hoit JD, Garshick E . Respiratory dysfunction and management in spinal cord injury. Respir Care 2006; 51: 853–870.
Manning HL, Shea SA, Schwartzstein RM, Lansing RW, Brown R, Banzett RB . Reduced tidal volume increases “air hunger” at fixed PCO2 in ventilated quadriplegics. Respir Physiol 1992; 90: 19–30.
Gutierrez CJ, Harrow J, Haines F . Using an evidenced-based protocol to guide rehabilitation and weaning of ventilator dependent cervical spinal cord injury patients. J Rehabil Res Dev 2003; 40: 99–110.
Acknowledgements
We thank the patients who volunteered to participate in this research. Gale Whiteneck, of the Craig Hospital Research Department participated in the conceptualization of the study design. Cate McGraw and Kelly Mowrey were actively involved in patient care and implementation of the research protocol. Matthew Fleishman, independently and blindly interpreted the chest radiographs. This research was supported by funding from the National Institute on Disability and Rehabilitation Research (NIDRR). The opinions here are those of the grantee and do not necessarily reflect those of the U.S. Department of Education, grant number H133N00006.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Fenton, J., Warner, M., Lammertse, D. et al. A comparison of high vs standard tidal volumes in ventilator weaning for individuals with sub-acute spinal cord injuries: a site-specific randomized clinical trial. Spinal Cord 54, 234–238 (2016). https://doi.org/10.1038/sc.2015.145
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2015.145