Abstract
Study design:
A prospective, animal model for pharmacological intervention of decompression sickness (DCS), including spinal cord (SC) injury.
Background:
Signs and symptoms of DCS can include joint pain, skin discoloration, cardiopulmonary congestion and SC injury; severity ranges from trivial to fatal. Non-recompressive therapy for DCS may improve time-to-treatment and therefore impact mortality and morbidity.
Objectives:
Oxycyte at 5 cc kg−1 provides both SC protection and statistically significant survival benefit in a swine model of DCS. The purpose of this study was to test whether a reduced dose of Oxycyte (3 cc kg−1) would provide similar benefit.
Setting:
Silver Spring, MD, USA
Methods:
Male Yorkshire swine (N=50) underwent a non-linear compression profile to 200 fsw (feet of sea water), which was identical to previous work using the 5 cc kg−1 dose of Oxycyte. After 31 min of bottom time, decompression was initiated at 30 fsw per minute until surface pressure was reached. Following decompression and the onset of DCS, intravenous Oxycyte or saline was administered with concurrent 100% O2 for 1 h. The primary end point was DCS-induced mortality, with Tarlov score and SC histopathology as secondary end points.
Results:
Oxycyte administration of 3 cc kg−1 following surfacing produced no significant detectable survival benefit. Animals that received Oxycyte, however, had reduced SC lesion area.
Conclusion:
Further studies to determine the lowest fully efficacious dose of Oxycyte for the adjunct treatment of DCS are warranted.
Similar content being viewed by others
Introduction
Decompression sickness (DCS) occurs when a decrease in ambient pressure exceeds a supersaturation state and allows release of inert gas from tissues. Though the pathophysiology of DCS is not fully characterized, there is general1 consensus that its occurrence is mediated by the presence of gas bubbles that result from this excess of inert gas. DCS is seen in hyperbaric conditions (diving) as well as in high-altitude conditions (pilots and astronauts).
In diving-related DCS, one concerning manifestation is neurologic injury, particularly in the spinal cord (SC-DCS). SC-DCS is a potentially devastating disease with a reported residual deficit noted in up to 33% of patients after treatment.2 Current standard treatment consists of recompression in a hyperbaric chamber as well as hyperbaric oxygen administration. However, the remote location of diving-related injuries, as well as the size, complexity and availability of a hyperbaric chamber can result in delays in treatment between 6 and 24 h.3
It appears that in severe DCS, a significant delay in therapy is associated with increased residual deficits.3 Specific to SC-DCS, Blatteau et al.4, described increased residual deficits in SC-DCS when recompression therapy was delayed by >6 h. In that work, residual deficits were noted in 30 out of 82 patients with a delay in recompression therapy as compared with 30 out of 124 patients who received recompression therapy within 3 h. One way to overcome such delays would be the use of so-called non-recompressive therapies. As a concept, so-called non-recompressive therapies are agents or strategies that treat DCS without the need of a hyperbaric chamber. As such, non-recompressive therapies for DCS hold the promise of delivering therapy at the time of injury as well as potentially negating the need for logistically burdensome recompression therapy. Such therapies would be particularly relevant to disabled submarine rescue, which presents multiple logistical challenges in a potential mass-casualty situation.
One promising candidate for the non-recompressive treatment of SC-DCS is intravenous perfluorocarbon (PFC). PFCs are fluorinated hydrocarbons that transport gases based on solubility alone. In the emulsified state, PFCs have a large solubility capacity for respiratory gasses (O2 (7.6 mM; 19.3 vol%), and CO2 (61.6 mM; 157 vol%)).5 This gas solubility of PFC, along with the small particle size (∼0.2 μm), allows PFC to overcome the relative resistance to gas flux imposed by plasma. As such, PFCs can enhance tissue oxygen delivery as well as maintain oxygen transport in low-flow states. In animal models of DCS, improved tissue oxygen delivery and enhanced gas elimination have been observed.6 As a class, PFCs have successfully prevented and treated DCS in several large and small animal models7, 8 and appear to decrease SC dysfunction seen in animal models of DCS.9
Recently, we demonstrated a decreased mortality by treating DCS in a 20-kg swine model with an intravenous dose of Oxycyte of 5 cc kg−1.10 Using methods similar to those described in that study, we hypothesized that a smaller dose of Oxycyte would still impart significantly decreased mortality benefits in the same swine model of severe DCS.
Materials and methods
All experiments were conducted according to the principles set forth in the ‘Guide for the Care and Use of Laboratory Animals,’ Institute of Laboratory Animal Resources, National Research Council, National Academy Press, 1996. Before commencing, our Institutional Animal Care and Use Committee reviewed and approved all aspects of this protocol. The institution’s animal care facility is fully AAALAC accredited and the veterinary staff members are familiar with our 20 kg swine model.
Statement of ethics
We certify that all applicable institutional and governmental regulations concerning the ethical use of animals were followed during the course of this research.
Animals
Male Yorkshire swine (N=50) from a single vendor (Thomas Morris Inc., Reisterstown, MD, USA) were housed in free running cages at our animal care facility where they acclimated for 5 days before any procedures. They were fed standard pig chow twice daily (2–2.5% bodyweight; Quality Lab Prod, Elkridge, MA, USA) with free access to water.
Treadmill training and use
SC-DCS is manifested as paresis/paralysis and sensory deficits.11 We incorporated the Tarlov Scale, a recognized standard, developed specifically for SC pathology in swine.12 Animals were trained to walk on a treadmill (T-2000, GE Healthcare, Milwaukee, WI, USA) in three sessions starting at least 2 days before hyperbaric exposure. Each session was defined as complete when the animal walked comfortably for 5 min at 1 mph, but never exceeded 15 min. Normal gait (Tarlov=5) was defined as walking at 1 mph for 5 min.
Hyperbaric exposure
We followed a DCS-inducing dive profile developed in our laboratory that produces a control mortality of ∼45% with evidence of SC pathology.10 On the day of the dive, paired awake, un-anesthetized swine were lead into individual standard wired dog transport cages (iCrate, MidwestPetProducts.com, NOLA Retail, Covington, LA, USA) that were then positioned inside the hyperbaric chamber (45 cu ft). Viewports were fitted with cameras aligned to observe and record the animals throughout the hyperbaric exposure. The chamber was sealed and pressurized using air.
The animals underwent a non-linear compression profile to 200 fsw. Bottom time was defined as the time from leaving surface pressure until time leaving bottom pressure. After 31 min of bottom time, decompression was initiated at 30 fsw per minute until surface pressure was reached and the chamber door opened.
Immediate post-dive procedures
The animals were taken out of the chamber, removed from their kennels and placed in a Panepinto sling. An ear-vein catheter was used to administer 0.25 mg kg−1 diazepam and animals were observed for up to 60 min for signs of cutis marmorata (‘skin bends’) as previously described.11
Treatment
At the onset of cutis marmorata, the animal was then given 100% O2 by snout cone (Smith Medical North America, Wausesha, WI, USA) and randomized to receive either 3 cc kg−1 intravenous Oxycyte (Oxygen Biotherapeutics, Inc., Morrisville, NC, USA) (n=27) or an equivalent dose of normal saline (n=23). Oxycyte is a third-generation PFC emulsion that has 60% weight/volume of perfluoro(t-butylcyclohexane). Animals were continuously observed, and any signs of distress, including thrashing or vocalization, were treated with additional diazepam (0.125 mg kg−1 up to 2 mg kg−1). Death was confirmed by the principle investigator. After 1 h, the nose cone was removed and the animals were returned to the holding pens.
24-h Assessment
All surviving animals (n=10 saline; n=13 Oxycyte) were assessed for their ability to stand 24 h post-dive. If able to stand, the animal was assigned a Tarlov score of 3 and placed onto the treadmill. Treadmill speed was then gradually increased in 0.2 mph increments over ∼30 s until the animal was able to walk at 1 mph for 5 min. This earned a Tarlov score of 5. If the animal was able to walk but unable to achieve 1 mph, the subject was scored a Tarlov 4 (weak walk). Other Tarlov scores were assigned as: complete paralysis of hind limbs—0; minimal movement of hind limbs—1; able to stand with assistance only—2. After this 24-h assessment, animals underwent euthanasia and SC fixation. Though a longer recovery time may be desired for histologic analysis, the 24-h time point was chosen to allow the greatest balance between histology assessment and comfort of a paralyzed animal.
Histology
Perfused-fixed SCs were trimmed at levels C5-6, T8-9 and L3-4; each cord section was split into five equal blocks that were mounted into a cassette before paraffin embedding such that each paraffin block would produce five coronal sections per slide when cut. Cord sections were processed through graded alcohols, embedded in paraffin, sectioned at 5 μm and stained with hematoxylin and eosin.
Lesion volume measurement
Bright-field photomicrographs of the stained slides were captured using an Olympus AX80 microscope (Olympus Corporation, Tokyo, Japan) equipped with a × 1.25 Olympus plan Apo objective and an Olympus DP70 digital camera. Photomicrographs were saved in tiff file format for analysis using imageJ64 (Rasband, W.S., ImageJ, US National Institutes of Health, Bethesda, MD, USA; http://imagej.nih.gov/ij/, 1997–2011) by an observer blinded to the nature of the groupings. Each SC image was opened using the ImageJ program and converted to grayscale. The SC section was then outlined using the polygon tool and the background was deleted using the clear outside function. The lookup tables were then inverted on the image so lesion areas were darkened. The threshold was then adjusted to predefined threshold settings, which were identical for all but three animals. The three animals that could not be analyzed with the preset threshold were analyzed with a user-defined threshold that most accurately outlined the lesions. The threshold selected area was then measured and divided by the total area of the section obtained by adjusting the threshold so that the marked area covered the entire section and repeating the measurement. A ratio of total aggregate lesion area over the entire section to total cross-sectional area for each trimmed SC section was determined. The ratios were then averaged from the five sections on each slide and converted into a mean area percentage for that SC region (cervical, thoracic, lumbar). The mean area percentages from all of the SCs in each group at each region were then compiled into an excel spreadsheet for downstream statistical analysis.
Statistics
All data were compiled into excel spreadsheets and then imported into Graphpad Prism 5 for statistical analysis (Graphpad Software, Inc., La Jolla, CA, USA). Mortality incidence was evaluated by a Fisher’s exact test and a two-sample, one-sided test for equality of proportions. Mean time to death, Tarlov score and Pre-dive weights were compared using two-tailed Student’s t-test. Mean SC lesion areas were compared using one-way analysis of variance with post hoc Bonferroni’s multiple comparisons test. Correlation between Tarlov score and lesion area was analyzed using Pearson’s correlation analysis.
Results
Weights and mortality
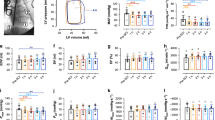
DCS incidence and severity is strongly influenced by subject weight. Mean animal weights per group were not significantly different (P=0.6846) prior to dive (Figure 1). There was no significant difference (P=0.6) between mortality incidence (Figure 2a), or time to mortality (P=0.7467) (Figure 2b).
Mortality incidence and mean time to death was not significantly decreased by Oxycyte treatment. Animals (N=50) were subjected to hyperbaric exposure then randomized to receive Oxycyte (3 cc kg−1) or Saline vehicle control after surfacing (a). Bars represent the number of animals that survived or died within each treatment group. There was no significant difference in mortality incidence (P=0.6) as determined by Fisher’s exact test with a two-sample one-sided test for equality of proportions. Mean time to death (b) was measured for each group and there were no significant differences (P=0.7467) between groups as determined by two-tailed Student’s t-test.
Pathology
Histological examination of tissue sections taken from cervical, thoracic and lumbar regions of the SCs of 24-h survivors was undertaken. SC-DCS injury was evident as large grossly apparent white matter lesions (Figure 3a) primarily concentrated in the cervical SC. In PFC-treated animals, the white matter lesions were smaller and less frequent (Figure 3b). Qualitative analysis of the observed white matter lesions revealed Wallerian degeneration, axonal swelling (Figure 3c1) and phagocytosis of damaged axons by macrophages/microglia (Figure 3c, 2, 3). Quantification of the total aggregate lesion area from the entire H&E-stained sections demonstrated a significant (P=0.002) decrease in cervical lesion area in Oxycyte-treated animals when compared with saline-treated controls (Figure 3d).
Spinal cord lesion area was significantly reduced by Oxycyte treatment. Spinal cords from swine that survived for 24 h were processed and stained as described in the Materials and methods section. Large hypo-intense lesions (areas of diminished staining) were primarily seen in the cervical SC of swine in the Saline group (a). Lesions from the same SC region in Oxycyte-treated animals (b) were much smaller or absent. Qualitative analysis of the white matter lesion ( × 40 magnification) demonstrated axonal swelling (c1), and immune cell infiltration of the myelin sheath (c2, 3). Quantification of the area of staining (d) revealed a significant decrease in lesion area in the cervical SCs of Oxycyte-treated animals when compared with Saline controls. Bars represent mean percent (%) lesion area ±s.e.m. Asterisk denotes P=0.002 as determined with one-way analysis of variance with post hoc Bonferroni’s multiple comparisons test.
Tarlov
There was no significant effect of Oxycyte on mean Tarlov scores (P=0.8176) (Figure 4a), nor was there a significant correlation between Tarlov score and SC lesion area (P=0.3333 and 0.5167 for NSS and Oxycyte, respectively) (Figure 4b).
Mean Tarlov score was not significantly altered by Oxycyte treatment and did not correlate with SC lesion area. Animals that survived to 24 h (n=23) were assessed as described in the Materials and methods section. There was no significant effect of Oxycyte on mean Tarlov scores (P=0.8176, two-tailed Student’s t-test) (a). Bars represent mean Tarlov score ±s.e.m. There was no significant correlation between Tarlov score and SC lesion volume (P=0.3333, and 0.5167 for NSS and Oxycyte, respectively, Pearson’s correlation) (b).
Discussion
The primary goal of this study was to evaluate whether Oxycyte at 3 cc kg−1 reduces mortality in a swine model of severe DCS. While the Oxycyte treatment did not show any decreased mortality or incidence of severe DCS, we did demonstrate mitigation of SC pathology. To our knowledge this is the first study to demonstrate significant SC tissue sparing using a non-recompressive adjunct therapy for DCS.
When neurologic injury occurs in hyperbaric DCS, the SC is affected in the majority of cases. The manifestation of SC injury induced by DCS ranges from minimal subjective sensory deficits to full paralysis and potentially death.13 Clinically, SC-DCS is heterogeneous and has a 20–30% chance of residual sequelae. Togawa et al.14 elegantly described evidence of non-overlapping neurologic findings (so-called dissociation) in 76 out of 101 patients with SC-DCS. More recently, Blatteau et al.4 cataloged over 200 divers with SC-DCS. In that review a higher initial injury score was associated with prolonged sequelae, which was observed in 28% of patients. Additionally, it appeared that in the most severely injured patients, a delay to recompression therapy may have been associated with a higher chance of permanent injury.4, 15
The pathophysiology of SC-DCS is yet to be clarified but contributing factors include venous stasis, arterial embolization and autochthonous bubble formation. Potentially, all of these hypothesized mechanisms would be improved with PFC.
PFCs carry gases solely based on their enhanced solubility. As they are immiscible with water, they must be emulsified in order to be used parenterally. In the emulsified state, the particles are ∼0.2 μm in diameter. It is likely enhanced oxygen solubility along with the small particle size allows PFCs to improve oxygen transport in conditions of relative low intravascular flow. Additionally, the enhanced solubility of nitrogen is likely responsible for the increased gas elimination that has been demonstrated with PFC in swine with experimentally induced DCS.6 Another therapeutic benefit of PFCs may be their intrinsic surfactant properties, which can protect the endothelial lining from known bubble-induced dysfunction.16 In one of the first experiments supporting the protective benefit of PFCs in neurologic manifestations of DCS, Dromsky demonstrated a survival benefit in 20 kg swine after an air-saturation dive when PFC was given immediately upon surfacing (before onset of DCS signs).7 In this work, the use of PFC completely protected the animals from central nervous system related DCS. Previously, we have demonstrated a survival benefit in 20 kg swine when the PFC Oxycyte was used as a treatment for DCS (after onset of DCS signs) and noted less evidence of SC injury.10 More recently, Zhang et al.9 demonstrated a protective effect of PFC in rat DCS SC injury as measured by somatosensory evoked potentials. Likewise, PFCs reduced mortality in experimentally induced systemic air embolism17 as well as in focal cerebral air embolism.18
Given the previous mortality benefit of a 5 cc kg−1 Oxycyte, the choice of a 3 cc kg−1 dose may come into question. Modern (so-called third generation) emulsified PFC have been studied in animal models of DCS in doses ranging from 4.5 to 7 ml kg−1 of 60% vol/wt emulsions.7, 9, 10 However in human trials involving disease states ranging from isovolemic hemodilution to traumatic brain injury, the highest dose studied has been 3 ml kg−1 (equating to 1.8 mg kg−1 of PFC). Despite evidence of therapeutic efficacy in animal models of DCS with several formulations of PFC, human studies involving DCS are lacking. As diving-related injuries are likely to occur sporadically and remote from available medical care, as well as ethical issues of potentially withholding the current gold standard therapy of HBO, human therapeutic field trials are not feasible. Thus, it is likely that animal models will serve as the best evidence for therapeutic efficacy in diving-related injury treated with PFC and was a large consideration in choosing a 3 ml kg−1 dose of Oxycyte.
This swine model of DCS with SC injury demonstrated that the cervical area of the SC was disproportionally affected. Hence, it is not surprising that this particular region of the SC appeared to benefit most from Oxycyte treatment, having the largest improvement in pathologic injury. Such regional variation has been reported in humans with SC-DCS and in animal models. In their rat model of SC-DCS, Marzella and Yin19 observed more lesions in the cervical area. Dick et al.20 later demonstrated that swine appeared to have increased abnormalities in the thoracic region. However, these findings may be subject to a selection bias, as areas of analysis were selected based on grossly affected injured areas at the time of necropsy. Regional variation in our previous work using 5 cc kg−1 Oxycyte is unknown as SC histopathology was only analyzed in a qualitative manner without objective regional quantification.10 In humans, SC injury appears predominantly localized to the upper third or middle thoracic segments.21
The lack of correlation between our functional assessment scale (Tarlov) and the amount of objective SC injury is unexpected. However, as described in swine previously,11 the SC injury in this model is associated with multifocal microscopic hemorrhages, largely located in the white matter. It is quite possible that specific location(s) of injury have a greater functional impact than the sheer volume of injury. The lack of correlation may also be attributable to the 5-level coarseness of the Tarlov scale in motor dysfunction and the fact that sensory dysfunction was not evaluated. Though the Tarlov scale has been shown to be a reliable measurement in focal SC injuries,12 its use has not been compared with other scales in SC-DCS.
The lack of any benefit to be demonstrated with a 3 cc kg−1 dose of Oxycyte on survival in this study is not wholly surprising. If gas delivery and elimination is in large part due to concentration of Oxycyte, this low dose may be inadequate to overcome the massive bubble load anticipated in this arduous diving profile. However, some incremental benefit on oxygen delivery, nitrogen elimination and surfactant properties that allows for reduced SC pathology would be expected. To our knowledge, in a large animal model, this is the smallest dose of any PFC studied. The absence of a detectable mortality decrease allowed us to gain insights into potential benefits of Oxycyte in neurologic DCS. The novel histological findings in this study were the result of experimental refinements derived from the previously published work studying Oxycyte at 5 cc kg−1.10 Based on the data presented here, further head-to-head comparative dosing studies utilizing Oxycyte to treat DCS in swine are warranted.
Conclusions
Oxycyte administration at a dose of 3 cc kg−1 in a swine model of SC-DCS, improves SC injury, but provides no detectable survival benefit. A previous study demonstrated that a higher dose of Oxycyte (5 cc kg−1) provided a statistically significant survival benefit.10 Together, these findings support the clinical potential for the use of Oxycyte as a non-recompressive treatment for DCS when standard recompression therapy is either unavailable or will be delayed. Further studies of the clinical utility of Oxycyte for DCS are warranted.
Data archiving
There were no data to deposit.
References
Madden LA, Laden G . Gas bubbles may not be the underlying cause of decompression illness – the at-depth endothelial dysfunction hypothesis. Med Hypotheses 2009; 72: 389–392.
Gempp E, Blatteau JE, Stephant E, Pontier JM, Constantin P, Peny C . MRI findings and clinical outcome in 45 divers with spinal cord decompression sickness. Aviat Space Environ Med 2008; 79: 1112–1116.
Ball R . Effect of severity, time to recompression with oxygen, and re-treatment on outcome in forty-nine cases of spinal cord decompression sickness. Undersea Hyperb Med 1993; 20: 133–145.
Blatteau JE, Gempp E, Simon O, Coulange M, Delafosse B, Souday V et al Prognostic factors of spinal cord decompression sickness in recreational diving: retrospective and multicentric analysis of 279 cases. Neurocrit Care 2011; 15: 20–27.
Zhu J, Hullett JB, Somera L, Barbee RW, Ward KR, Berger BE et al Intravenous perfluorocarbon emulsion increases nitrogen washout after venous gas emboli in rabbits. Undersea Hyperb Med 2007; 34: 7–20.
Spiess BD, Zhu J, Pierce B, Weis R, Berger BE, Reses J et al Effects of perfluorocarbon infusion in an anesthetized swine decompression model. J Surg Res 2009; 153: 83–94.
Dromsky DM, Spiess BD, Fahlman A . Treatment of decompression sickness in swine with intravenous perfluorocarbon emulsion. Aviat Space Environ Med 2004; 75: 301–305.
Spiess BD, McCarthy RJ, Tuman KJ, Woronowicz AW, Tool KA, Ivankovish AD . Treatment of decompression sickness with a perfluorocarbon emulsion (FC-43). Undersea Biomed Res 1988; 15: 31–37.
Zhang RJ, Lui K, Kang ZM, Fan DF, Ni XX, Liu Y et al Combined effects of intravenous perfluorocarbon emulsion and oxygen breathing on decompression-induced spinal cord injury in rats. Undersea Hyperb Med 2011; 38: 335–343.
Mahon RT, Watanabe TT, Wilson MC, Auker CR . Intravenous perfluorocarbon after onset of decompression sickness decrease morality in 20-kg swine. Aviat Space Environ Med 2010; 81: 555–559.
Broome JR, Dick EJ . Neurological decompression illness in swine. Aviat Space Environ Med 1996; 67: 207–213.
Tarlov I . Spinal Cord Compressions: Mechanisms of Paralysis and Treatment. Charles C Thomas: Springfield, IL, 147 1957.
Blick G . Notes on diver’s paralysis. Br Med J 1909; 2: 1796–1798.
Togawa S, Maruyama M, Yamami N, Nakayama H, Shibayama M, Kawashima M et al Dissociation of neurological deficits in spinal decompression illness. Undersea Hyperb Med 2006; 33: 265–270.
Ball R, Survanshi S . Outcome from neurological decompression illness. Aviat Space Environ Med 1997; 6: 654–658.
Torres LN, Spiess BD, Torres FIP . Tissue oxygenation and microvascular hemodynamics in experimental arterial gas embolism. Undersea Hyperb Med 2011; 38: 537–548.
Spiess BD, Braverman B, Woronowicz AW, Ivankovich AD . Protection from cerebral air emboli with perfluorocarbons in rabbits. Stroke 1986; 17: 1146–1149.
Spiess BD, McCarthy RJ, Piotrowski D, Ivankovich AD . Protection from venous air embolism with fluorocarbon emulsion FC-43. J Surg Res 1986; 41: 439–444.
Marzella L, Yin A . Role of extravascular gas bubbles in spinal cord injury induced by decompression sickness in the rat. Exp Mol Pathol 1994; 61: 16–23.
Dick EJ, Broom JR, Hayward IJ . Acute neurologic decompression illness in pigs: lesions of the spinal cord and brain. Lab Anim Sci 1997; 47: 50–57.
Kei PL, Choong CT, Young T, Lee SH, Lim CC . Decompression sickness: MRI of the spinal cord. J Neuroimaging 2007; 17: 378–380.
Acknowledgements
The views expressed in this article are those of the author and do not necessarily reflect the official policy or position of the Department of Navy, Department of Defense, nor the US Government. This work was funded by the Office of Naval Research work unit #603792 N.02914.W050.A0710. The authors are US Government employees and this work was prepared as part of their official duties. Title 17 U.S.C. provides that copyright protection is not available for work prepared as a part of official duties.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Mahon, R., Auker, C., Bradley, S. et al. The emulsified perfluorocarbon Oxycyte improves spinal cord injury in a swine model of decompression sickness. Spinal Cord 51, 188–192 (2013). https://doi.org/10.1038/sc.2012.135
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2012.135
Keywords
This article is cited by
-
Current perspectives of artificial oxygen carriers as red blood cell substitutes: a review of old to cutting-edge technologies using in vitro and in vivo assessments
Journal of Pharmaceutical Investigation (2023)
-
Recent advances in the pharmacologic treatment of spinal cord injury
Metabolic Brain Disease (2015)