Abstract
Study design:
Prospective, experimental study.
Objective:
To investigate whether the heart rate (HR) at lactate minimum (LMHR)determined by means of a HR-guided lactate minimum test (LMT) corresponds to the HR at maximal lactate steady state (MLSS) in wheelchair-racing athletes.
Setting:
Institute of Sports Medicine.
Methods:
Eight well-trained wheelchair-racing athletes (mean age: 33±12 years; height: 169±12 cm; body mass: 59±11 kg; V̇O2peak: 2.76±0.73 l min−1) completed a HR-guided LMT on a treadmill in their own racing wheelchair. Subsequently, exercise intensity at MLSS and corresponding HR were determined by means of several endurance tests on different days.
Results:
All measured parameters (HR, speed, blood lactate, oxygen consumption and rating of perceived exertion) revealed significantly higher values at MLSS compared with values at lactate minimum (LM). However, there were highly significant correlations (r=0.914, P=0.002) between LMHR and HR at MLSS (163±6 versus 172±7 b.p.m.) as well as for speed (r=0.935, P=0.001) and blood lactate (r=0.944, P<0.001) at LM versus MLSS. The correlation for oxygen consumption (r=0.798, P=0.018) at LM versus MLSS was lower yet significant.
Conclusions:
There exists a close relationship between LMHR and HR at MLSS in wheelchair racing. This allows the prediction of MLSS based on a single exercise test in this special group of athletes. For practical use during daily training, routine HR at MLSS can be assumed to be 8–9 b.p.m. above the LMHRin wheelchair-racing athletes.
Similar content being viewed by others
Introduction
The maximal lactate steady state (MLSS) is a common predictor of endurance exercise capacity and a helpful tool to guide training intensities.1, 2, 3 MLSS is defined as the highest exercise intensity that can be sustained over time without continuous blood lactate accumulation.4 Thereby, blood lactate should not increase >1 mmol l−1 during the last 20 min of a 30-min constant load endurance test.5, 6, 7 To accurately determine MLSS, several exercise tests at different intensities and on different days are necessary, making this method impractical.5 However, there is some evidence from the literature that exercise intensity at MLSS can be determined by a single test—the so-called lactate minimum test (LMT) in patients as well as in moderately and well-trained athletes.8, 9, 10, 11
Besides the fact that the LMT would be a time-saving method (because only a single test is necessary) to determine MLSS, it also offers further advantages. Test results seem to be independent of the previous nutritional status12 or of the investigator's experience.11 In combination with an ergospirometric assessment, maximal oxygen consumption can be concomitantly determined during an LMT as well.13 In summary, the LMT seems to be an easy and objective tool providing accurate and helpful results not only for scientific purposes but also for athletes and coaches in their daily practice,11 at least for running and cycling exercise in able-bodied subjects.
In general, an LMT consists of two parts: During the first part, high-intensity exercise is performed to induce severe lactic acidosis. The second part corresponds to an incremental test starting at a moderate aerobic exercise intensity. Under these aerobic conditions during the early phase of the second part, blood lactate is metabolized with increasing workloads until a lactate minimum (LM) is reached. LM denotes the intensity at which an equilibrium between blood lactate appearance and elimination is established. Thereafter, blood lactate concentration begins to rise again.
Recently, our group developed a new, standardized and user-friendly heart rate (HR)-guided LMT protocol, which was found to be a highly reproducible method for cycling exercise in able-bodied subjects.14 However, there exists no study so far that used the LMT for exercise testing in wheelchair athletes. Additionally, it seems difficult to transfer the present knowledge from LMTs with able-bodied subjects into such a special group of disabled athletes. In this context one has to bear in mind that compared with able-bodied individuals, athletes with a spinal cord injury have unique changes in metabolic, cardiorespiratory, neuromuscular and thermoregulatory systems, which reduce their overall physical capacity.15 These disability-related physiological changes may lead to different test results using the HR-based LMT in wheelchair athletes. Thus, the aim of the present study was to investigate the precise relationship between HR at MLSS and that at LM in competitive wheelchair-racing athletes based on our HR-guided LMT protocol.14
Methods
Subjects
Eight well-trained wheelchair athletes (seven men, one woman) participated in the study. The subject characteristics and paralympic racing classification can be found in Table 1. The study was approved by the local ethics committee. Written informed consent was obtained from each subject (for the minor, consent was obtained from his parents) before the start of the study. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
Subjects were asked not to perform strenuous workouts the day before each test. Training and nutrition on the days before the test and on the test days were kept constant and recorded.
Although some subjects had a lesion level above the sixth thoracic vertebral segment, we did not observe any influence on HR regulation because of constraints in sympathetic heart innervations (for peak HR data see Table 1), which enabled us to apply the proposed HR-guided protocol14 (for protocol details see below) in all subjects without any adaptations.
Testing protocol
Part A: Lactate minimum test (LMT)
Subjects completed a HR-based LMT in their own racing wheelchair on a motor-driven treadmill (HP Cosmos Saturn, HP Cosmos, Traunstein, Germany) according to the following protocol described in detail by Strupler et al.14 Briefly, HR was recorded after sitting in the wheelchair for 2 min (HR at rest). After a 5-min warm-up period at 10 km h−1, the LMT began with the protocol described by Conconi et al.,16 starting at 12 km h−1. At every 200 m, treadmill speed was increased by 0.5 km h−1. The incline of the treadmill was set at 2% and subjects received verbal encouragement to perform until exhaustion. Peak HR at the end of the Conconi test was defined as maximal HR.
The second part of the LMT started immediately at the end of the Conconi test and was guided by the subject's individual HR. For each stage a target HR was calculated. Target HR for the first stage was calculated from HR at rest plus 60% of HR reserve, which was determined by subtraction of HR at rest from the maximal HR, meeting the guidelines for aerobic training.17 For the following stages the increments of HR were 8% of the HR reserve, but with a maximal augmentation of 8 b.p.m. Treadmill speed was adjusted to reach the predicted HR within 3 min and to keep it constant (±2 b.p.m.) for the last 2 min of the stage. Mean HR during the last minute of each stage was used for analysis. The first stage of the second part of the LMT lasted 7 min and the following stages lasted 5 min each. The test was terminated when blood lactate concentration started to rise again by at least 0.2 mmol l−1. On average, a HR-guided LMT lasted about 40 min, whereas normally five stages during the second part of the LMT were performed until LM could be determined.
For blood lactate measurements, 20 μl of blood was taken from the earlobe and analyzed immediately by an enzymatic method (Super GL Ambulance, Ruhrtal Labor Technik, Möhnesee, Germany). Blood lactate was measured at rest, at the end of the first part and at the end of each stage of the second part of the LMT while the subjects were exercising. HR was monitored continuously (Polar S610i, Polar Electro, Kempele, Finland) during the LMT; rating of perceived exertion according to Borg18 was acquired using the 15-point scale ranging from 6 (no exertion) to 20 (maximal exertion). Oxygen uptake (Oxycon Pro, Jaeger GmbH, Hoechberg, Germany) was determined breath by breath, whereas 15-s-averaged values were used for data analysis. Treadmill speed was recorded continuously during the LMT. The test was terminated when blood lactate concentration increased by >0.2 mmol l−1 compared with the previous stage. HR of the stage before blood lactate increased again (LM) was defined as HR at lactate minimum (LMHR).
Part B: Endurance tests to determine maximal lactate steady state (MLSS)
Based on LMHR, the MLSS (represented by maximal constant HR) was determined by several endurance tests on different days. All endurance tests of the same subject were performed at the same time of the day in order to exclude circadian performance fluctuations. Each endurance test started with a standardized warm-up of 3 min at 60% of the speed at LM (determined in the LMT described above in Part A), 3 min at 80% and 4 min at 100% of the speed at LM. Subsequently, treadmill speed was increased until the predetermined target HR was reached within 5 min. Thereafter, target HR was kept constant (±2 b.p.m., corresponding to a range of 4 b.p.m.) for 30 min. If necessary, treadmill speed was adequately adjusted to keep HR within these narrow limits. Treadmill incline was again set at 2%.
Blood lactate samples were drawn at rest, after 3, 6 and 10 min of warm-up, as well as subsequently every 5 min until the end of the endurance test. Blood lactate analysis followed immediately after blood sampling. Rating of perceived exertion was always recorded immediately before blood lactate sampling. HR as well as ventilatory and gas exchange variables were measured continuously throughout the test.
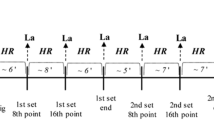
Target HR for the first endurance test was set at 8 b.p.m. above LMHR. If blood lactate concentration did not increase by >1 mmol l−1 during the last 20 min of the endurance test, HR intensity was increased by another 4 b.p.m. (=LMHR+12 b.p.m.) for the second endurance test (Figure 1). In case of a lactate steady state at this intensity, target HR was again adapted in each subsequent endurance test by 4 b.p.m. until MLSS was determined.
If blood lactate increased by >1 mmol l−1 during the last 20 min of the first endurance test or if a subject was not able to complete the endurance test at an intensity 8 b.p.m. above LMHR, target HR for the next endurance test was reduced by 4 b.p.m. (Figure 1). This study design allowed us to determine the MLSS for each subject after 2–3 endurance tests.
Statistics
For statistical analysis a commercially available software package (SPSS 13, Chicago, IL, USA) was used. Data are presented as means±s.d. Mean values at LM and MLSS were compared using a paired-sample t-test. The coefficients of correlation were calculated using Pearson's correlation matrix. The level of significance was set at P<0.05. In order to show the difference between data at LM and at MLSS, Bland and Altman plots were used19 to compare the difference between MLSS and LM (bias) versus their average value.
Results
All measured parameters (HR, blood lactate concentration, rating of perceived exertion, oxygen uptake (V̇O2) and treadmill velocity) were significantly lower at LM compared with MLSS (Table 2). The corresponding Bland and Altman plots revealed a positive bias for each parameter mentioned, whereas all data with the exception of one data point were within the limits of agreement.
All subjects were able to complete the first endurance test without a blood lactate increase of >1 mmol l−1 during the last 20 min of the test. Thereafter, seven out of eight subjects were not able to complete the second endurance test at an intensity of 12 b.p.m. above LMHR and the remaining subject aborted the third endurance test at 16 b.p.m. above LMHR.
There was a significant correlation between LMHR and HR at MLSS (r=0.914; P=0.002; Figure 2), but HR at MLSS was significantly higher than LMHR (172±7 versus 163±6 b.p.m.; P<0.001). The mean difference (bias) for LMHR and HR at MLSS, and the limits of agreement (mean±2 s.d. of the difference) are graphically presented in Figure 3 by means of a Bland and Altman plot. The corresponding bias for HR was 8±3 b.p.m. LMHR and HR at MLSS were found to be 88±2% and 92±2% of the maximal HR, respectively.
Peak blood lactate concentration at the end of the first part of the LMT was 8.5±2.3 mmol l−1, absolute peak oxygen consumption (V̇O2peak) 2.76±0.73 l min−1, maximal treadmill speed 21.2±4.0 km h−1 and the corresponding rating of perceived exertion 19±1.
Significant correlations between values at LM and MLSS were found for treadmill speed (r=0.935, P=0.001), blood lactate concentrations (r=0.944; P<0.001) and V̇O2 (r=0.798, P=0.018). V̇O2 at LM and at MLSS were 67±7% and 76±9% of the V̇O2peak, respectively.
Discussion
The main finding of the present study was that all measured values at LM determined by a HR-guided LMT were significantly below the values at MLSS (Table 2). These findings are in line with the results of other studies that used workload-guided LMT protocols.9, 11
However, we found a close and significant correlation between values at LM and those at MLSS for HR data (Figure 2), which allows the prediction of HR and thus exercise intensity at MLSS in our wheelchair-racing athletes. These findings support the role of HR as one of the main predictors of training intensity, especially during exercise in the field.20, 21 This is of practical importance, as transferring laboratory-based absolute data such as speed or workload often is difficult,22 even more in wheelchair racing. Further, the application of HR as a feasible tool for guidance of endurance training was also confirmed by results of a recently published study22 showing a high and reproducible correlation between maximal constant HR and MLSS. This result is in line with observations of sports practice, where athletes perform at a constant HR during competitions (for example, cycling time trial performance)23, 24 rather than at constant blood lactate concentrations or V̇O2. Results of a study by Janssen et al.25 investigating male handcycle users seem to support these findings also for subjects with spinal cord injury. Keeping this in mind, HR seems to be a useful and easy-to-use parameter to guide training intensity even in wheelchair-racing athletes with intact sympathetic heart innervation. However, to determine different HR intensity zones for a systematic guidance of the endurance training, the knowledge of HR at and related to MLSS seems to be crucial. Based on the present findings, it seems possible to gain this information for a group of highly trained wheelchair-racing athletes of the paralympic racing categories T53 and T54 based on a single LMT, which helps to facilitate the daily business of athletes, coaches and scientists.
Additionally, the use of an LMT seems to offer further advantages compared with other common exercise tests applied. LMT results seem to be independent of the previous nutritional status12 and of the investigator's experience, as test results do not depend on a subjective estimation of, for example, ‘thresholds’.11 Further, in combination with an ergospirometric assessment, maximal oxygen consumption can be concomitantly determined during the first part of an LMT as well.13 In summary, the LMT seems to be an easy and objective test providing accurate and helpful test results not only for scientific purposes but also for the daily work of athletes and coaches.11
However, although the above-mentioned advantages seem to be valid for the LMT in general, one has to keep in mind that at present several different LMT protocols are applied. This circumstance might lead to slightly different outcomes depending on the test protocol used (for example, workload-guided versus HR-guided protocols). Several previous studies8, 9, 10, 12 have found that power output at LM corresponded to the definition of MLSS when performing a constant workload test, whereas a slightly higher power output resulted in increasing blood lactate concentrations throughout the test. This observation stands in contrast to the results presented in our study. However, all the above-mentioned studies used a workload-guided protocol, whereas our study was based on a HR-guided one.14 This means that with our test protocol the workload during the second part of the LMT is continuously adjusted to reach the predetermined HR and to maintain it within narrow limits. This stands in contrast to workload-guided protocols, where a constant workload for each stage is kept, which may lead to a HR drift.
Moreover, in the present study, subjects transitioned immediately into the incremental phase after the high-intensity loading phase during the first part of the LMT. In contrast, most of the other studies included a rest or recovery period of several minutes before starting with the incremental phase.7, 8, 9, 10, 11, 12 This difference in methodology might have led to different lactate kinetics resulting in significantly lower values at LM compared with MLSS (Table 2) in the present study. Further, the increments of 8 b.p.m. for each stage during the second part of the LMT might also have resulted in a certain inaccuracy, as such an increment might be overspending. In this context, a modification of the LMT test protocol towards smaller and/or shorter increments during the second part of the LMT might be helpful, but first has to be validated by further investigations. However, if successful, such an adaptation should result in the outcome that values at LM ideally correspond to values at MLSS. Such a finding would further improve the quality and accuracy of the HR-guided LMT.
Finally, one has to bear in mind that our study investigated wheelchair athletes performing upper-body exercise, whereas the results of former studies were based on cycling or running data in able-bodied subjects, where a higher total muscle mass is involved during exercise. One could hypothesize that the amount of muscle mass involved might influence the relation between HR at LM compared with MLSS. However, further studies are needed to prove this hypothesis.
Conclusions
There exists a close relationship between LMHR and HR at MLSS in wheelchair-racing athletes, but also for other parameters such as treadmill speed and blood lactate concentration. Consequently, the prediction of MLSS based on a single HR-based LMT in this special group of athletes can be obtained. If training is guided based on HR recommendations during the daily training routine, HR at MLSS can be assumed to be 8–9 b.p.m. above LMHR in wheelchair-racing athletes.
References
Haverty M, Kenney WL, Hodgson JL . Lactate and gas exchange responses to incremental and steady state running. Br J Sports Med 1988; 22: 51–54.
Heck H, Mader A, Hess G, Mucke S, Muller R, Hollmann W . Justification of the 4-mmol/l lactate threshold. Int J Sports Med 1985; 6: 117–130.
Kindermann W, Simon G, Keul J . The significance of the aerobic-anaerobic transition for the determination of work load intensities during endurance training. Eur J Appl Physiol Occup Physiol 1979; 42: 25–34.
Beneke R . Anaerobic threshold, individual anaerobic threshold, and maximal lactate steady state in rowing. Med Sci Sports Exerc 1995; 27: 863–867.
Beneke R, Hutler M, Leithauser RM . Maximal lactate-steady-state independent of performance. Med Sci Sports Exerc 2000; 32: 1135–1139.
Billat VL, Sirvent P, Py G, Koralsztein JP, Mercier J . The concept of maximal lactate steady state: a bridge between biochemistry, physiology and sport science. Sports Med 2003; 33: 407–426.
Jones AM, Doust JH . The validity of the lactate minimum test for determination of the maximal lactate steady state. Med Sci Sports Exerc 1998; 30: 1304–1313.
Bacon L, Kern M . Evaluating a test protocol for predicting maximum lactate steady state. J Sports Med Phys Fitness 1999; 39: 300–308.
MacIntosh BR, Esau S, Svedahl K . The lactate minimum test for cycling: estimation of the maximal lactate steady state. Can J Appl Physiol 2002; 27: 232–249.
Tegtbur U, Machold H, Meyer H, Storp D, Busse MW . Determining the extent of intensive physical performance in patients with coronary heart disease. Z Kardiol 2001; 90: 637–645.
Knoepfli-Lenzin C, Boutellier U . Lactate minimum is valid to estimate maximal lactate steady state in moderately and highly trained subjects. J Strength Cond Res 2011; 25: 1355–1359.
Tegtbur U, Busse MW, Braumann KM . Estimation of an individual equilibrium between lactate production and catabolism during exercise. Med Sci Sports Exerc 1993; 25: 620–627.
Dantas De Luca R, Rocha R, Burini RC, Coelho Greco C, Denadai BS . The lactate minimum test protocol provides valid measures of cycle ergometer VO2peak. J Sports Med Phys Fitness 2003; 43: 279–284.
Strupler M, Mueller G, Perret C . Heart rate based lactate minimum test—a reproducible method. Br J Sports Med 2009; 43: 432–436.
Bhambhani Y . Physiology of wheelchair racing in athletes with spinal cord injury. Sports Med 2002; 32: 23–51.
Conconi F, Grazzi G, Casoni I, Guglielmini C, Borsetto C, Ballarin E et al. The Conconi test: methodology after 12 years of application. Int J Sports Med 1996; 17: 509–519.
American College of Sports Medicine. Exercise prescription. In: Franklin BA, Whaley MH, Howley ET (eds). ACSM’ Guidelines for Exercise Testing and Prescription, 6th edn. Lippincott Williams & Wilkins: Baltimore, 2000, pp 135–164.
Borg GA . Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982; 14: 377–381.
Bland JM, Altman DG . Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1: 307–310.
Foster C, Crowe MP, Holum D, Sandvig S, Schrager M, Snyder AC et al. The bloodless lactate profile. Med Sci Sports Exerc 1995; 27: 927–933.
Röcker K, Striegel H, Dickhuth HH . Heart-rate recommendations: transfer between running and cycling exercise. Int J Sports Med 2003; 24: 173–178.
Vobejda C, Frommer K, Samson W, Zimmermann E . Maximal constant heart rate—a heart rate based method to estimate maximal lactate steady state in running. Int J Sports Med 2006; 27: 368–372.
Hoogeveen AR, Hoogsteen J, Schep G . The maximal lactate steady state in elite endurance athletes. Jpn J Physiol 1997; 47: 481–485.
Palmer AS, Potteiger JA, Nau KL, Tong RJ . A 1-day maximal lactate steady-state assessment protocol for trained runners. Med Sci Sports Exerc 1999; 31: 1336–1341.
Janssen TWJ, Dallmeijer AJ, van der Woude LHV . Physical capacity and race performance of handcycle users. J Rehabil Res Dev 2001; 38: 33–40.
Acknowledgements
This study was financially supported by the Swiss Olympic Association.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Perret, C., Labruyère, R., Mueller, G. et al. Correlation of heart rate at lactate minimum and maximal lactate steady state in wheelchair-racing athletes. Spinal Cord 50, 33–36 (2012). https://doi.org/10.1038/sc.2011.97
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2011.97
Keywords
This article is cited by
-
Blood lactate and ventilatory thresholds in wheelchair athletes with tetraplegia and paraplegia
European Journal of Applied Physiology (2014)
-
Field-Based Physiological Testing of Wheelchair Athletes
Sports Medicine (2013)