Abstract
Study design:
Non-randomized study.
Objectives:
Previous studies indicated that at least 2-h leg exercise at more than 60% maximum oxygen consumption (VO2max) increased plasma interleukin (IL)-6 in able-bodied (AB) subjects. The purpose of the present study was to compare IL-6 response to arm exercise in AB subjects and persons with spinal cord injury (SCI).
Setting:
Wakayama Medical University in Japan.
Methods:
Six subjects with SCI between T6 and T10 and seven AB subjects performed 2-h arm crank ergometer exercise at 60%VO2max. Plasma catecholamines, IL-6, tumor necrosis factor (TNF)-α and high-sensitivity C-reactive protein (hsCRP) were measured before exercise, 60-min exercise, immediately and 2 h after the completion of exercise.
Results:
Arm exercise increased myoglobin and plasma IL-6 levels in SCI and AB (P<0.01), but there were no differences in them between the two groups throughout the study. Plasma levels creatine kinase, lactate dehydrogenase, TNF-α and hsCRP did not change throughout the study in both groups.
Conclusion:
These findings suggest neither significant muscle damage nor inflammatory response during exercise. The increase in plasma IL-6 in SCI was not unexpected, confirming that moderate intensity and relatively long-arm exercise is safe and beneficial for SCI subjects with regard to IL-6 excretion, as in AB subjects.
Similar content being viewed by others
Introduction
Leg muscle contraction in able-bodied persons (AB) results in marked production of interleukin (IL)-6 from myocytes and its release in the circulation, and such production is independent of muscle damage.1 Identification of IL-6 production by skeletal muscles during physical activity generated renewed interest in the metabolic role of IL-6. Epidemical studies show tumor necrosis factor (TNF)-α and IL-6 increase in persons with obesity and life style-related diseases;1 however, the increase of IL-6 is inhibitory response against increased TNF-α.1 Therefore, during resting condition, serum IL-6 levels directly correlate with obesity and reduced insulin action.1 Although IL-6 is often classified as a pro-inflammatory cytokine, experimental evidence suggests that a rapid rise in circulating IL-6 enhances lipid oxidation, improves insulin-stimulated glucose uptake and has anti-inflammatory effects.1 These results suggest that exercise-induced IL-6 production likely mediates some of the beneficial effects of exercise in chronic diseases.
Today, the majority of persons with spinal cord injury (SCI) complete their rehabilitation program, return to society and enjoy sports activities. However, it is well known that SCI per se is associated with a low-grade chronic inflammatory state, a small increase in plasma IL-6, increased risk of atherosclerosis, coronary heart disease and type 2 diabetes.2, 3 Where most AB individuals perform some combination of upper and lower body movements, really any sports performance in SCI is achieved by upper extremities only. As the quantity of muscle mass in the upper extremities is different, it is assumed that the IL-6 response to exercise is different to that of AB subjects. To our knowledge, however, there is no information on IL-6 response to exercise in SCI. In AB subjects, arm crank exercise does not result in increase of serum IL-6 probably due to the involvement of a limited muscle mass.4 Apart from muscle mass, the magnitude of the exercise-induced increase in plasma IL-6 is determined by the combination of mode, intensity and duration of the exercise. One previous studies indicated that the exercise-induced IL-6 response is sensitive to exercise intensity and seems to require at least 60% of maximum oxygen consumption (VO2max).5, 6 Furthermore, the duration of exercise (2 h) and exercise intensity are significant determinants of plasma IL-6 concentrations during endurance leg exercise in AB subjects.1, 7, 8 In the previous studies, the protocol of the well-fixed endurance exercise study to find the differences between AB and SCI was 2-h arm exercise at 60%VO2max.9 Therefore, any comparative study between SCI and AB subjects should be carried out under identical optimal exercise duration and intensity.9
The purpose of present study was to compare the IL-6 response and changes in other inflammatory markers between SCI and AB subjects during arm crank exercise of well-controlled intensity and duration.
Materials and methods
Subjects
The study included six SCI subjects who were involved in a regular physical training program. We also included seven AB as control subjects. The characteristics of the participating subjects are presented in Table 1. There were no differences between SCI and AB with respect to age, height, weight and VO2max. The selection criteria were the following: (1) men with history of SCI between Th6 and Th10. (2) Intact motor activity of the upper limbs and genitourinary and intestinal functions. (3) Excellent health at the time of the study and no medications that would affect the immune or endocrine systems. Written informed consent was obtained from all participants, and the study protocol was approved by the Human Research Committee of our hospital.
Study protocol
Before 2 weeks the scheduled start of the study, subjects performed a progressive VO2max test on the arm crank ergometer (818E, Monark, Sweden). The test protocol required the subjects to maintain a target cadence of 60 r.p.m. After a 15-min rest period, subjects performed unloaded exercise for 3 min. This was followed by increasing the power output by 20 W every 3 min. After reaching 60 W, the power output was increased by 10 W every minute. The test was terminated when the subjects reached exhaustion and Borg Scale of 20, or the cadence fell below 60 r.p.m. O2 uptake and ventilation were measured with respiratory metabolic cart (model AERO300S, Minato Mediac Science, Co., Tokyo, Japan). The electrocardiogram was recorded with electrocardiogram monitor (model ML-9000, Fukuda Denshi, Co., Tokyo, Japan). All subjects were allowed to drink water throughout the study.
All subjects indicated that they had avoided intensive exercise for at least 24 h before the test and they were healthy and free of symptoms associated with respiratory and urinary tract infections. The subjects had their regular dinner before 2200 hours the day before and then refrained from eating but were allowed to drink tap water ad libitum until the start of the study. They reported to the Human Performance Laboratory at 1000 and were outfitted with electrodes for electrocardiogram recording. During the 1-h rest-phase, the subject sat quietly in a chair but was allowed to read or watch non-stimulating books and movies; however, they were discouraged from sleeping. Subsequently, the subject started to exercise on an arm crank ergometer for 2 h at an intensity of 60% VO2max and the power output was increased progressively from 0 W to the set level over a 3-min period.
Blood samples were collected from the antecubital vein using heparinized tubes and ethylenediaminetetraacetic acid-2K containing tubes before exercise, 60 min of exercise, immediately after exercise and 2 h following the completion of exercise. The total blood volume in each sampling period was 6 ml (0.5 ml for IL-6, 1.5 ml for muscle enzymes, 2 ml for adrenaline and cortisol and 2 ml for blood cell counts). The subjects underwent 5-h rest examination as a time control. Blood samples were collected at five points that corresponded with the exercise examination. The time control and the exercise test were performed 1 week apart in no special order. Seven AB subjects performed the same experiment as the control group.
IL-6 assay
Blood samples for IL-6 measurement were drawn into glass tubes containing ethylenediaminetetraacetic acid. The tubes were spun immediately at 3500 × g for 15 min at 4 °C. The plasma was stored at −80 °C until analysis. High-sensitivity chemiluminescent enzyme immunoassay kit (Fujirebio Co., Tokyo, Japan) was used for measurement of IL-6 concentration in plasma (sensitivity: 0.2 pg ml−1). Each measurement was performed in duplicate.
Other blood tests
Total blood cell count was determined using a cell counter. Hematocrit (Hct) was measured by centrifugation. Enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN, USA) was used to measure plasma TNF-α concentration. Serum high-sensitivity C-reactive protein (hsCRP) levels were measured by a latex-enhanced immunonephelometric assay (Dade Behring, Marburg, Germany). Plasma concentration of myoglobin was measured using a commercially available radioimmunoassay (TFB Co., Tokyo, Japan). Creatine kinase (CK) and lactate dehydrogenase activity in plasma was measured by using a commercial kit (Kanto Chemical Co., Tokyo, Japan). Catecholamines were extracted from plasma using alumina and measured by high-performance liquid chromatography using a modification of the procedure described by Hunter et al.10 Plasma cortisol levels were assayed using a competitive solid phase125I radioimmunoassay technique (Dainabot Lab, Tokyo, Japan).
Statistical analysis
Data were expressed as mean±s.d. Data of Table 1 were analyzed using the Student's t-test. Differences between data recorded before exercise and each time point after exercise, and between two groups and conditions we analyzed by Wilcoxon Mann–Whitney test. A P-value less than 0.05 denoted the presence of a significant difference between two groups.
Results
Changes in blood cell count
Hct increased (P<0.05) during the 60-min exercise and immediately after exercise in SCI and AB, but returned to baseline level 2 h after exercise in both groups (Table 2). Leukocyte count increased (P<0.05) immediately after exercise, reached a plateau and then remained at that level for 2 h after exercise in both SCI and AB.
Changes in IL-6
Plasma IL-6 significantly increased during, immediately after and at 2 h after exercise in SCI and AB (Figure 1). There were no differences in plasma IL-6 between the two groups before exercise, during exercise, immediately after exercise and at 2 h after exercise.
Changes in TNF-α and hsCRP
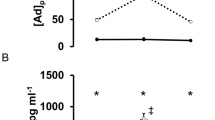
Plasma TNF-α and hsCRP did not change throughout the study in SCI and AB (Figure 2). However, plasma hsCRP was significantly higher in SCI than AB during exercise, after exercise and at 2 h after exercise.
Changes in myoglobin, CK and lactate dehydrogenase levels
Arm crank ergometer exercise significantly increased myoglobin levels immediately and at 2 h after exercise in the SCI and AB (Figure 3). However, there were no changes in CK and lactate dehydrogenase during and after exercise in both groups.
Changes in catecholamine and cortisol levels
Plasma adrenaline levels were higher during and immediately after exercise in SCI and AB relative to the baseline (Figure 4). Plasma adrenaline immediately after exercise in AB was significantly higher than in SCI. Plasma noradrenaline increased during and immediately after exercise in both groups. Plasma cortisol did not change during rest or exercise in SCI and AB.
Discussion
The major findings of the present study were that 2-h arm crank ergometer exercise at 60%VO2max was associated with 1) a significant increase in plasma IL-6 in both SCI and AB, but not with any changes in TNF-α or hsCRP, 2) a significant increase in plasma catecholamines during and after exercise in both SCI and AB, and 3) a significant increase in myoglobin in SCI and AB. It is possible that the source of increased IL-6 during arm crank exercise was the inflammatory response during exercise11, 12 and/or increased production/secretion by the contracting skeletal muscles. In this regard, Pedersen et al.13 indicated that the exercise-related increase in IL-6 was mainly induced by IL-6 gene transcription in the contracting skeletal muscles. To our knowledge, the present study is the first to demonstrate that 2-h arm crank ergometer exercise at 60%VO2max increased IL-6 but not TNF-α and hsCRP in SCI, and the results are in agreement with the above study.13 Importantly, the stable levels of TNF-α and hsCRP throughout the study suggest a lack of inflammatory response. In other words, the increase in IL-6 during arm crank exercise does not reflect an inflammatory response during exercise, and there is a safe and effective exercise modality for SCI.
The majority of previous studies on IL-6 response to exercise used leg exercise. Febbraio et al.14 demonstrated eightfold increase in plasma IL-6 after 2-h leg cycle exercise at 62±2% VO2max in six healthy AB. However, arm exercise does not seem to increase plasma IL-6 to levels above those before the exercise in AB.1 The present study demonstrated that the 2-h arm crank exercise, which did not involve muscles of the lower extremities, increased plasma IL-6. The intensity of VO2max applied in the present study was similar to that applied by Febbraio et al.;14 however, our results showed a threefold increase in IL-6 relative to the pre-exercise level in SCI. This finding suggests that arm crank exercise is sufficient to induce IL-6 secretion.
Another possible source of the increased IL-6 during exercise in SCI is the adipose tissue. In fact, adipose tissue is one of the major sources of IL-6,15 and exercise results in increased IL-6 gene expression in adipose tissue.15 Furthermore, our experience suggests that SCI subjects often have relatively larger proportion of adipose tissue than AB subjects.
Some patients with SCI have sympathetic nervous system impairment and thereby reduced catecholamine response to exercise compared with AB subjects.16 The present results showed significant increases in plasma adrenaline and IL-6 concentrations during exercise in SCI and AB, although the magnitude of the increase in adrenalin levels during arm crank exercise in SCI was smaller than in AB. These findings suggest that the participating SCI subjects did have sympathetic nervous system abnormalities. It has been suggested that adrenaline may stimulate IL-6 gene transcription of protein kinase A via β-adrenergic stimulation;17 however, the present results did not support the IL-6 gene transcription effect in adrenalin.
In the present study, the increase in myoglobin during arm crank ergometer exercise at 60%VO2max might be induced by increase in sarcolemmal permeability and not muscle damage.18 The stable levels of CK compared with the increase in myoglobin level may be due to the difference in molecular weight of the two proteins; a smaller molecular size of CK (85 000 Da) compared with myoglobin (18 000 Da).
Delayed peak IL-6 level
In the present study, the peak IL-6 level occurred 2 h after exercise in SCI and AB. In previous studies of lower-body cycling exercise, the peak plasma IL-6 level was noted immediately after 3 h exercise at 50% VO2max5 and at 2.5 h exercise at 75% VO2max19 followed by a rapid fall toward pre-exercise levels. These results indicate that arm crank exercise induces changes in plasma IL-6 kinetics that are different from those of lower-body cycling exercise. Hiscock et al.20 reported that the source of IL-6 was the myocytes in exercising leg muscles at 55%VO2 max. Therefore, we assumed the increase of IL-6 during arm exercise might come from contracting arm muscles; however, the mechanism of IL-6 production during arm crank exercise is not clear at present. Therefore, the different time course of IL-6 secretion in the arm crank exercise suggests that the different type of myocytes of upper arm muscles promoted IL-6 production from the contracting muscle. Further studies are needed to determine the mechanism involved in the delayed peak level of IL-6 in arm exercise, compared with leg exercise.
High basal hsCRP in SCI
Higher levels of hsCRP were noted in SCI than AB throughout the study. This finding suggests a chronic inflammatory state in SCI. In this regard, Wang et al.2 demonstrated significantly elevated serum levels of CRP in SCI, irrespective of injury duration and injury level, compared with AB. The results of the present study add support to the above study. However, the basal level of IL-6 in SCI was similar to that of AB, suggesting that the low-grade chronic inflammatory state did not induce an increase in basal IL-6.
Study limitation
The present study found no significant differences in plasma IL-6 levels between the SCI and AB groups. Careful examination of the values showed large standard deviation of IL-6 values in both groups, denoting differences among the individuals of the same group. We surveyed more than 100 persons with SCI, but selected only a few subjects for the study who fulfilled the inclusion criteria. Apart from the large standard deviation, the present study showed that 2-h arm exercise at 60% VO2max resulted in a significant increase in IL-6 in both AB and SCI subjects.
Conclusion
The present study demonstrated that 2-h arm crank ergometer exercise at 60%VO2 max resulted in a significant increase in IL-6 in SCI, but not in other inflammatory markers. The results suggest that arm exercise does not induce acute inflammatory response and that moderate intensity and relatively long-arm exercise is a safe and beneficial for SCI subjects with regard to plasma IL-6 levels.
References
Pedersen BK, Febbraio MA . Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev 2008; 88: 1379–1406.
Wang TD, Wang YH, Huang TS, Su TC, Pan SL, Chen SY . Circulating levels of markers of inflammation and endothelial activation are increased in men with chronic spinal cord injury. J Formos Med Assoc 2007; 106: 919–928.
Bauman WA, Spungen AM . Coronary heart disease in individuals with spinal cord injury: assessment of risk factors. Spinal Cord 2008; 46: 466–476.
Bergfors M, Barnekow-Bergkvist M, Kalezic N, Lyskov E, Eriksson JW . Short-term effects of repetitive arm work and dynamic exercise on glucose metabolism and insulin sensitivity. Acta Physiol Scand 2005; 183: 345–356.
Fischer CP, Hiscock N, Basu S, Vessby B, Kallner A, Pedersen BK et al. Supplementation with vitamins C and E inhibits the release of interleukin-6 from contracting human skeletal muscle. J Physiol 2004; 558: 633–645.
Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Klarlund PB . Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol 2000; 529: 237–242.
Fischer CP . Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc Immunol Rev 2006; 12: 6–33.
Ostrowski K, Schjerling P, Pedersen BK . Physical activity and plasma interleukin-6 in humans: effect of intensity of exercise. Eur J Appl Physiol 2000; 83: 512–515.
Ueta M, Furusawa K, Takahashi M, Akatsu Y, Nakamura T, Tajima F . Attenuation of natural killer cell activity during 2-h exercise in individuals with spinal cord injuries. Spinal Cord 2008; 46: 26–32.
Hunter LW, Rorie DK, Yaksh TL, Tyce GM . Concurrent separation of catecholamines, dihydroxyphenylglycol, vasoactive intestinal peptide, and neuropeptide Y in superfusate and tissue extract. Anal Biochem 1998; 173: 340–352.
Bruunsgaard H, Galbo H, Halkjaer-Kristensen J, Johansen TL, MacLean DA, Pedersen BK . Exercise-induced increase in serum interleukin-6 in humans is related to muscle damage. J Physiol 1997; 499: 833–841.
Nehlsen-Canarella SL, Fagoaga OR, Nieman DC, Henson DA, Butterworth DE, Davis JM et al. Carbohydrate and the cytokine response to 2.5 h of running. J Appl Physiol 1997; 82: 1662–1667.
Keller C, Steensberg A, Pilegaard H, Osada T, Saltin B, Pedersen BK et al. Transcriptional activation of the IL-6 gene in human contracting skeletal muscle: influence of muscle glycogen content. FASEB J 2001; 15: 2748–2750.
Febbraio MA, Ott P, Nielsen HB, Steensberg A, Keller C, Pedersen BK et al. Hepatosplanchnic clearance of interleukin-6 in humans during exercise. Am J Physiol Endocrinol Metab 2003; 285: E397–E402.
Keller C, Keller P, Marshall-Gradisnik SM, Pedersen BK . IL-6gene expression in human adipose tissue in response to exercise: effect of carbohydrate ingestion. J Physiol 2003; 550: 927–931.
Kjaer M, Pollack SF, Mohr T, Weiss H, Gleim GW, Ragnarsson KT et al. Regulation of glucose turnover and hormonal responses during electrical cycling in tetraplegic humans. Am J Physiol 1996; 271: R191–R199.
Febbraio MA, Pedersen BK . Contraction-induced myokine production and release: is skeletal muscle an endocrine organ? Exerc Sport Sci Rev 2005; 33: 114–119.
Kuipers H . Exercise-induced muscle damage. Int J Sports Med 1994; 15: 132–135.
Ostowski K, Hermann C, Bangash A, Schjerling P, Nielsen JN, Pedersen BK . A trauma-like elevation of plasma cytokines in humans in response to treadmill running. J Physiol 1998; 513: 889–894.
Hiscock N, Chan MH, Bisucci T, Darby IA, Febbraio MA . Skeletal myocytes are a source of interleukin-6 mRNA expression and protein release during contraction: evidence of fiber type specificity. FASEB J 2004; 18: 992–994.
Acknowledgements
We are grateful to Drs Midori Yamanaka, Masaki Goto, Motohiko Banno, Tomoya Shimomatsu, Tomoyuki Ito and Takehiko Baba for the clinical assistance. We thank Mrs Hiroyasu Uenishi, Takahiro Miyake, Tokio Kinoshita, Kenzou Tearamura, Makoto Kawanishi, Hironobu Tanaka, Takasi Moriki, Atsuhiro Sugino, and Yoshio Yamamoto, and Ms. Yumi Koike, Keiko Sakamoto and Kaori Hisada for their technical assistance. We also thank Dr Faiq G Issa, for the careful reading and editing of the manuscript. This project was supported by the Japanese National. Foundation for Scientific Research and Nachi Katsuura Research Found.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Umemoto, Y., Furusawa, K., Kouda, K. et al. Plasma IL-6 levels during arm exercise in persons with spinal cord injury. Spinal Cord 49, 1182–1187 (2011). https://doi.org/10.1038/sc.2011.74
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2011.74
Keywords
This article is cited by
-
Role of exercise on visceral adiposity after spinal cord injury: a cardiometabolic risk factor
European Journal of Applied Physiology (2021)
-
Methodological Considerations Which Could Improve Spinal Cord Injury Research
Journal of Science in Sport and Exercise (2020)
-
Can intervals enhance the inflammatory response and enjoyment in upper-body exercise?
European Journal of Applied Physiology (2017)
-
Elevation of interleukin-6 and attenuation of tumor necrosis factor-α during wheelchair half marathon in athletes with cervical spinal cord injuries
Spinal Cord (2014)
-
Increase in interleukin-6 immediately after wheelchair basketball games in persons with spinal cord injury: preliminary report
Spinal Cord (2013)