Abstract
Study design:
Observational cross-section study.
Objective:
The objective of our study was to determine if phosphorylation of aggregated neurofilaments (NFs) would occur in autoimmune-mediated motor neuron injury. Our main hypothesis was that autoimmune-mediated damage of spinal cord motor neurons may influence NF phosphorylation and lead to NF aggregation.
Methods:
A total of 20 guinea pigs were inoculated with bovine spinal cord anterior horn homogenates (experimental autoimmune gray matter model) and 20 guinea pigs were inoculated with phosphate-buffered saline (control). NF phosphorylation and aggregation were observed by immunohistochemistry and electron microscopic examination. Data were analyzed using Student's t-test with P<0.05 being considered significant.
Results:
Abnormal phosphorylation and distribution of NF occurred in motor neurons and axons of animals with experimental autoimmune gray matter disease but not in the control animals.
Conclusion:
Aberrant accumulation and phosphorylation of neurofilaments in perikarya of spinal cord motor neurons occur in immune-mediated motor neuron death. As both immunologic response and alteration of neurofilaments are observed in amyotrophic lateral sclerosis (ALS) patients and aberrant neurofilament change harms motor neurons, our present findings suggest that autoimmunity-induced ALS may mediate in part through neurofilament modification.
Similar content being viewed by others
Introduction
Amyotrophic lateral sclerosis (ALS) is an adult-onset and heterogeneous neurological disorder that affects primarily motor neurons of brain stem and spinal cord, resulting in paralysis and death within a few years. The mechanism of selective motor neuron killing in ALS remains understood, although many hypotheses have been proposed.1, 2 The etiology of ALS is believed to be multifactorial, and some mechanisms are known to be related to one another, for example, oxidative damage being a consequence of excitotoxicity.3, 4
A number of studies have implicated autoimmunity in ALS.5, 6, 7, 8, 9 Moreover, an experimental autoimmune gray matter disease (EAGMD) in guinea pigs, which is induced by inoculating the animals with bovine spinal cord ventral horn homogenate, presents with damage of both upper and lower motor neurons.10 These findings suggest that autoimmunity may have a major role in ALS development. In this report, we demonstrate that there is an increased accumulation and phosphorylation of neurofilament (NF) in motor neurons of animals with EAGMD, indicating that autoimmunity may lead to the aberrant change of NF.
Materials and methods
Animals
The experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals, from the National Research Council of China (1998). Male albino outbred Hartley guinea pigs were purchased from Weitonglihua Co. (Beijing, China). The animals were housed in the animal facility of the Second Hospital of Hebei Medical University.
The EAGMD model
EAGMD was induced using a published protocol.10 Briefly, 20 guinea pigs (4-month old, weighing 675–800 g) were inoculated with bovine spinal cord anterior horn homogenates. Fresh bovine spinal cord tissues were homogenized in phosphate-buffered saline (PBS) and administered to each animal at 0.26–0.37 g kg−1 body weight in 0.5 ml PBS and 0.5 ml complete Freund's adjuvant (Sigma Chemical Co., St Louis, MO, USA). The mixture was inoculated subcutaneously to 4–6 sites in the back of each animal. The treatment was repeated once a month for a total of four times or stopped when the animal developed signs of muscle weakness. The spinal cord homogenates were administered in incomplete Freund's adjuvant (Sigma) after the first inoculation. Altogether 10 control animals were sham treated, that is, the spinal cord homogenate was substituted with an equal volume of PBS. All animals were checked daily for speed and steadiness of movement, ability to maintain posture in upright position, response to a pinching stimulus, presence of impaired sphincter function and skin lesions, and breathing and swallowing functions. The animals were weighed weekly. Each animal also underwent electromyography (EMG) examination in the hind extremities using concentric needle electrode before each inoculation. When EMG indicated motor neuron dysfunction or muscle denervation in an animal, immunization to that animal was halted. Animals were killed when becoming moribund or 7 months after the first inoculation, including the two nonsymptomatic animals in the treatment group and all animals in the control group in the latter case. Before killing, the animals were anesthetized with chloral hydrate for blood drawing through retro-orbital puncture and intracardiac perfusion with 0.1 M phosphate buffer (pH 7.4) containing 0.05% glutaraldehyde and 4% paraformaldehyde. Spinal cords were then removed for examination of spinal cord motor neurons and processes. Blood specimens were used for measurement of serum titer of antimotor neuron antibodies.
Immunohistochemistry
Spinal cord was removed from each animal following intracardiac perfusion as described before. Cervical and lumber enlargements were cut and fixed in 0.1 M phosphate buffer (pH 7.4), containing 0.05% glutaraldehyde and 4% paraformaldehyde. The tissues were then cut into 30-μm sections using a vibratome (Leica VT1000S; Leica, Heidelberg, Germany). Free-floating tissue sections were rinsed with 50 mM Tris-buffered saline (TBS, pH 7.4), and the endogenous peroxidase was bleached with 3% H2O2 in 0.01 M PBS for 15 min. The tissue sections were then treated with 0.4% Triton X-100 in TBS for 30 min at room temperature and blocked for 1 h in TBS containing 10% normal horse serum and 0.1% Triton X-100. The sections were incubated with a primary antibody at 4 °C overnight, followed by incubation with a biotinylated horse secondary antibody for 2 h and finally treatment with an ABC mix (Golden Bridge Biotechnology, Zhongshan, Beijing, China) for 1 h. SMI-32 and SMI-31 antibodies (Sternberger Immunochemicals, Baltimore, MD, USA) were used to label nonphosphorylated and phosphorylated NF, respectively. The resultant peroxidase labeling was visualized by incubating the antibody-treated tissue sections with 0.005% diaminobenzidine and 0.01% H2O2 in 50 mM Tris-HCl (pH 7.6) for 10 min. The reaction was stopped by rinsing the sections with Tris-HCl buffer. The tissue sections were then mounted on slides, air-dried, dehydrated in graded alcohols, cleared in xylene and coverslipped with neutral balsam. The tissue sections were also processed with the primary antibody replaced with PBS to assess background staining. The immunohistochemical staining was recorded using a Nikon microscope (Labophot-2, Japan) equipped with a Nikon digital camera (Coolpix 950).
Motor neurons in the spinal cord sections were counted based on immunochemical staining.
Electron microscopic examination
The lumbar enlargements were cut transversely into approximately 1-mm thick slices. The ventral horn was excised and fixed in 0.1 M phosphate buffer (pH 7.4) containing 4% glutaraldehyde for one week at 4 °C, which was followed by treatment with 1% osmium tetroxide for 1 h at room temperature. The specimens were then washed in 0.01 M PBS, dehydrated with acetone and then embedded in epoxy resin. Semithin (∼1 μm thickness) sections were cut and stained with toluidine blue for examination by light microscope to determine tissue orientation. Ultrathin sections (70 nm) were then cut and stained with lead citrate and uranyl acetate for examination by a transmission electron microscope (JEM-1230, Joel, Japan).
Statistical analysis
Data were analyzed using Student's t-test with P<0.05 being considered significant.
Results
EAGMD was induced after inoculation of bovine spinal cord anterior horn homogenate
Of the 20 guinea pigs, 17 (85%) inoculated with bovine spinal cord anterior horn homogenate developed EAGMD. The onset time of EAGMD varied significantly, ranging from 31 days to 150 days after the first immunogen inoculation. For the remaining three guinea pigs, one died of accidental suffocation during the experimental period, whereas two guinea pigs did not develop the disease during the 7-month experimental period. The symptomatic animals underwent progressive muscle weakness and atrophy, finally leading to respiratory failure and death within 13±17 days (mean±s.d.). EMG with concentric needle electrode indicated muscle denervation (data now shown). Moreover, serum titer of antibodies against spinal cord motor neurons in animals with EAGMD ranged from 1:400 to 1:12 800, whereas the titers in control animals were lower than 1:100.
The number of spinal cord anterior horn motor neurons in cervical enlargement and lumbar enlargement in the animals with EAGMD decreased 44.2 and 47.2%, respectively.
Abnormal phosphorylation and distribution of NF in motor neurons and axons of animals with EAGMD
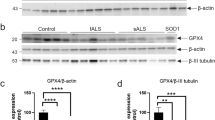
In the control animals, motor neurons in the spinal cord were strongly stained for NF using SMI-32 (Figure 1a). In contrast, in animals with EAGMD, not only was the number of motor neurons in the spinal cord decreased, but also NF staining in the remaining motor neurons was significantly weaker (Figure 1b), in line with a previous observation.11 Phosphorylated NF (p-NF) was weakly stained using SMI-31 in neuron of control spinal cord (Figure 1c). However, p-NF staining increased markedly in the spinal cord motor neurons of EAGMD animals (Figure 1d).
Levels of neurofilament (NF) and phosphorylated neurofilament (p-NF) in spinal cord motor neurons. Sections of spinal cord (lumbar enlargement) from a control animal (a, c) and an animal with experimental autoimmune gray matter disease (EAGMD) (b, d) were stained immunohistochemically for both NF (a and b, using an SMI-32 antibody) and p-NF (c, d, using an SMI-31 antibody). The images are representative of spinal cord motor neurons from other animals in the same group.
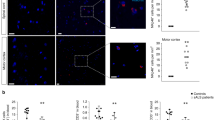
Moreover, in spinal cord of control animals, NF was present in low levels and loosely distributed in perikarya, and NFs in neuronal axon were arranged in parallel along the processes (Figures 2a and b). In contrast, in EAGMD animals NF was aggregated in bundles in neuron perikarya (Figures 2c and d).
Ultrastructural changes of spinal cord motor neurons and processes in animals with experimental autoimmune gray matter disease (EAGMD). The images were obtained with transmission electron microscopy (TEM × 4000–15 000). (a) The cytoplasm of a normal spinal cord motor neuron, (b) a normal neuronal process, (c) the cytoplasm of a spinal cord motor neuron of an EAGMD animal (the arrow points to an aggregate of neurofilaments, NFs) and (d) neuronal processes of an EAGMD animal.
Discussion
Our present study has shown that guinea pigs after inoculation with bovine spinal cord anterior horn homogenate develop neurological deficit, motor neuron loss and NF alteration. These results add new information to the EAGMD animal model previously reported by Engelhardt et al.10
NFs are major intermediate filaments in neurons and consist mainly of three subunits, including light-chain (NF-L, 62 kDa), middle-chain (NL-M, 95 kDa) and highest-chain (neurofilament heavy subunit (NF-H), 110 kDa).11, 12 In a normal neuron, NFs are sparsely and irregularly aligned in perikarya, and are abundant and organized in parallel bundles in neuronal axons, are poorly phosphorylated in the perikarya but are highly phosphorylated in the axons. However, in a number of neurodegenerative diseases, including ALS, NF-H and HF-M are abnormally phosphorylated in the perikarya.13, 14, 15, 16 In the present study, we found markedly increased NF phosphorylation in the perikarya (Figure 1). Increased phosphorylation of NF has been reported to cause aberrant accumulation of NF in motor neurons and to slow NF transport through axon.17, 18 The mechanism by which increased NF phosphorylation takes place in these animals has not been elucidated. Cyclin-dependent kinase 5 (cdk5) was previously reported to phosphorylate NF-H.14, 19 Our preliminary study also indicated an increased level of cdk5 in spinal cord motor neurons of EAGMD animals (Y-L Liu et al., unpublished data). Examination of spinal cord tissues by transmission electron microscopy showed that NFs aggregated in bundles in perikarya of EAGMD animals (Figure 2). This finding is consistent with the notion that increased NF phosphorylation leads to aberrant accumulation of NF.
Aberrant NF phosphorylation and accumulation have been reported to occur in motor neurons of ALS patients and in a mouse model of ALS.18 Transgenic mice overexpressing human NF exhibited degeneration of motor neurons with pathologic features reminiscent of those found in ALS.20 Moreover, autoimmunity has been detected in ALS patients. In the context of these data, our present study suggests that immune-mediated NF modification may play a significant part in ALS development in humans.
In conclusion, aberrant accumulation and phosphorylation of neurofilament in perikarya of spinal cord motor neurons occur in immune-mediated motor neuron death. As both immunologic response and alteration of neurofilaments are observed in ALS patients and aberrant neurofilament change harms motor neurons, our present findings suggest that autoimmunity-induced ALS may mediate in part through neurofilament modification.
References
Cluskey S, Ramsden DB . Mechanisms of neurodegeneration in amyotrophic lateral sclerosis. Mol Pathol 2001; 54: 386–392.
Shaw PJ . Molecular and cellular pathways of neurodegeneration in motor neurone disease. J Neurol Neurosurg Psychiatry 2005; 76: 1046–1057.
Coyle JT, Puttfarcken P . Oxidative stress, glutamate, and neurodegenerative disorders. Science 1993; 262: 689–695.
Liu B, Gao HM, Wang JY, Jeohn GH, Cooper CL, Hong JS . Role of nitric oxide in inflammation-mediated neurodegeneration. Ann NY Acad Sci 2002; 962: 318–331.
Appel SH, Stockton-Appel V, Stewart SS, Kerman RH . Amyotrophic lateral sclerosis. Associated clinical disorders and immunological evaluations. Arch Neurol 1986; 43: 234–238.
Engelhardt JI, Appel SH . IgG reactivity in the spinal cord and motor cortex in amyotrophic lateral sclerosis. Arch Neurol 1990; 47: 1210–1216.
Engelhardt JI, Appel SH, Killian JM . Experimental autoimmune motoneuron disease. Ann Neurol 1989; 26: 368–376.
Kimura F, Smith RG, Delbono O, Nyormoi O, Schneider T, Nastainczyk W et al. Amyotrophic lateral sclerosis patient antibodies label Ca2+ channel alpha 1 subunit. Ann Neurol 1994; 35: 164–171.
Smith RG, Hamilton S, Hofmann F, Schneider T, Nastainczyk W, Birnbaumer L et al. Serum antibodies to L-type calcium channels in patients with amyotrophic lateral sclerosis. N Engl J Med 1992; 327: 1721–1728.
Engelhardt JI, Appel SH, Killian JM . Motor neuron destruction in guinea pigs immunized with bovine spinal cord ventral horn homogenate: experimental autoimmune gray matter disease. J Neuroimmunol 1990; 27: 21–31.
Gotow T . Neurofilaments in health and disease. Med Electron Microsc 2000; 33: 173–199.
Julien JP . Neurofilament functions in health and disease. Curr Opin Neurobiol 1999; 9: 554–560.
Tsang YM, Chiong F, Kuznetsov D, Kasarskis E, Geula C . Motor neurons are rich in non-phosphorylated neurofilaments: cross-species comparison and alterations in ALS. Brain Res 2000; 861: 45–58.
Bajaj NP, al-Sarraj ST, Leigh PN, Anderson V, Miller CC . Cyclin dependent kinase-5 (CDK-5) phosphorylates neurofilament heavy (NF-H) chain to generate epitopes for antibodies that label neurofilament accumulations in amyotrophic lateral sclerosis (ALS) and is present in affected motor neurones in ALS. Prog Neuropsychopharmacol Biol Psychiatry 1999; 23: 833–850.
Strong MJ . Neurofilament metabolism in sporadic amyotrophic lateral sclerosis. J Neurol Sci 1999; 169: 170–177.
Strong MJ, Kesavapany S, Pant HC . The pathobiology of amyotrophic lateral sclerosis: a proteinopathy? J Neuropathol Exp Neurol 2005; 64: 649–664.
Ackerley S, Thornhill P, Grierson AJ, Brownlees J, Anderton BH, Leigh PN et al. Neurofilament heavy chain side arm phosphorylation regulates axonal transport of neurofilaments. J Cell Biol 2003; 161: 489–495.
Rouleau GA, Clark AW, Rooke K, Pramatarova A, Krizus A, Suchowersky O et al. SOD1 mutation is associated with accumulation of neurofilaments in amyotrophic lateral sclerosis. Ann Neurol 1996; 39: 128–131.
Grant P, Sharma P, Pant HC . Cyclin-dependent protein kinase 5 (Cdk5) and the regulation of neurofilament metabolism. Eur J Biochem 2001; 268: 1534–1546.
Julien JP, Cote F, Collard JF . Mice overexpressing the human neurofilament heavy gene as a model of ALS. Neurobiol Aging 1995; 16: 487–490; discussion 490–482.
Acknowledgements
We thank Ms Bing Li for assistance in data analysis and article reading. This study was supported by a grant from the Science and Technology Committee of Hebei Province of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, YL., Guo, YS., Xu, L. et al. Alternation of neurofilaments in immune-mediated injury of spinal cord motor neurons. Spinal Cord 47, 166–170 (2009). https://doi.org/10.1038/sc.2008.90
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2008.90
Keywords
This article is cited by
-
Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes
Nature Neuroscience (2019)