Abstract
Background:
Bone mineral density (BMD) of the lumbar spine (L-spine) has been reported to be normal or increased in persons with chronic spinal cord injury (SCI).
Objective:
To determine BMD of the L-spine by dual-energy X-ray absorptiometry (DXA) and quantitative computerized tomography (qCT) in men with chronic SCI compared with able-bodied controls.
Design:
Cross-sectional, comparative study.
Setting:
Clinical research unit, Veterans Affairs Medical Center, Bronx, NY, USA and Kessler Institute of Rehabilitation, West Orange, NJ, USA.
Methods:
Measurements of the L-spine were made in 20 men with SCI and compared with 15 able-bodied controls. The DXA images were acquired on a GE Lunar DPX-IQ. The qCT images of the L-spine were acquired on a Picker Q series computerized tomographic scanner.
Results:
The mean ages for the SCI and control groups were 44±13 vs 42±9 years, and the duration of injury of the group with SCI was 14±11 years. There were no significant differences between the SCI and control groups for L-spine DXA BMD (1.391±0.210 vs 1.315±0.178 g/m2) or for L-spine DXA T-score (1.471±1.794 vs 0.782±1.481). L-spine qCT BMD was significantly lower in the SCI compared with the control group (1.296±0.416 vs 1.572±0.382 g/m2, P=0.05); the T-score approached significance (−1.838±1.366 vs −0.963±1.227, P=0.059). Subjects with moderate degenerative joint disease (DJD) had significantly higher T-scores by DXA than those without or with mild DJD.
Conclusion:
Individuals with SCI who have moderate to severe DJD may have bone loss of the L-spine that may be underestimated by DXA, reducing awareness of the risk of fracture.
Similar content being viewed by others
Introduction
Osteoporosis is a predictable occurrence below the level of injury in individuals with chronic spinal cord injury (SCI), especially of the long bones that predispose to fracture.1, 2, 3, 4, 5, 6 Of note, the literature is replete with reports of cross-sectional studies that have found, contrary to the extremities and other bony structures, that the vertebral column does not seem to lose bone mass after paralysis but, rather, tends to gain bone mass with age and/or longer duration of injury.1, 7, 8, 9, 10, 11 Various mechanisms have been proposed to explain why the vertebral spine does not lose bone mass, and these include continued application of gravity-related forces, the effects of prolonged seated posture and a different mechanical function of the spine than that of the limbs.
Two reports have previously challenged the notion that the vertebral bodies are spared from bone loss after paralysis.12, 13 Both studies have suggested that the absence of osteoporosis of the spine is a spurious finding of dual-energy X-ray absorptiometry (DXA).12, 13 Because of its facile application and low level of radiation exposure, DXA has been widely used to determine bone mineral density (BMD) of the hip and lumbar spine (L-spine) in the general population, and it has generally permitted the prompt and reproducible diagnosis of osteoporosis. However, the anteroposterior (AP) determination of the L-spine bone mass has been recognized to include in its field, when present, the measurement of extraneous calcification. In the general able-bodied population, extra-vertebral calcification may obscure the diagnosis of osteoporosis.14, 15, 16 DXA may also overestimate BMD of the L-spine in persons with SCI because of the presence of neuropathic calcification.12, 13
Imaging the spine with qCT permits the observer to restrict the field of interest to the mid-vertebrae, rather than the surrounding structures. Thus, qCT holds the potential to permit differentiation between the L-spine medullary portion of trabecular BMD from that of regions repleted with cortical bone and proximate areas in persons with SCI, which may spuriously elevate bone mass measurement. The ability to differentiate spuriously increased from actually decreased spine BMD is of importance as any exercise prescription that increases the forces on vertebrae that are osteoporotic should be avoided to reduce the risk of compression fracture. In persons with SCI and able-bodied controls, we compared two imaging techniques of the L-spine: DXA and quantitative computerized tomography (qCT).
Methods
This was a cross-sectional and comparative study. In the able-bodied controls, height and weight were measured in the standing position. In the group with SCI, height was measured while lying in the supine position and weight was measured on a Weight-Tronix scale (Scale Electronics Development, New York, NY, USA). Body mass index (BMI) was calculated as body weight in kilograms (kg) divided by height in meters squared (m2).
Subjects
Twenty male subjects with SCI (10 with paraplegia and 10 with tetraplegia) who were available for study (for example, convenience sample) and 15 age- and height-matched, able-bodied men who served as controls were recruited for study. Subjects with SCI were recruited from SCI Service of the James J Peters Veterans Affairs Medical Center, Bronx, NY, USA and the Kessler Institute of Rehabilitation, West Orange, NJ, USA; controls were recruited from the able-bodied veteran population, general community and hospital staff. The ethnic distribution in the SCI group was 7 Caucasians, 8 African–Americans and 5 Latinos; in the control group, the ethnic distribution was 1 Caucasian, 11 African–Americans, 1 Latino and 2 Asians. Participants were excluded from the study if they had a history of earlier bone disease (for example, Paget's disease, hyperparathyroidism, osteoporosis, heterotopic ossification, etc.) or any condition predisposing to osteoporosis (for example, alcohol abuse, anabolic or glucorticoid steroid administration, hyperthyroidism, Cushing's disease or syndrome and severe underlying chronic disease), other than SCI. Persons with surgical hardware of the spine were excluded from study participation. There was no attempt to include or exclude persons with DJD of the spine from study participation. Institutional review approval was obtained from the James J Peters Veterans Affairs Medical Center and the Kessler Institute of Rehabilitation before study participation.
DXA and qCT imaging
Each subject had a single DXA scan performed. The DXA images were acquired on a GE Lunar DPX-IQ, with the region of interest (ROI) (L-spine) isolated for quantification of regional BMD (Lunar Model DPX, version 3.6, Madison, WI, USA).17 The scans were acquired by trained technicians. Using a phantom, scanned 38 consecutive times over a 12-month period, day-to-day DXA variation was <1% (C.V.=0.06); in 15 subjects who were not participants in this study, day-to-day variability was found to be <1.5% (C.V.=1.493±0.014; 95% C.I.: lower=1.11%, upper=1.9%). Measurements for DXA were carried out on the same day or within 1 week of the qCT scan. Software algorithms were applied to isolate the ROI (Lunar instrumentation manual).15 The L-spine (L1 to L4 region) was displayed graphically and the operator adjusted the final-cut lines on each subject. All cuts were performed by the same technician to avoid inter-rater variability.
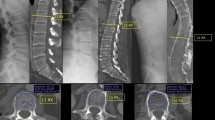
The subjects were studied with the Picker Q-Series computerized tomographic (CT) Scanner and Q-Series Bone Mineral Analysis Program (Q-BMAP) using the Q-BMAP work station for analysis in which the BMD value (mg/cm of equivalent bone density) was calculated. The technique captures BMD of medullary portion of trabecular bone of the L-spine, excluding cortical regions, as well as excluding regions of potential osteophyte formation and vascular calcification. Each subject had a single qCT scan performed. The subject was centered on the qCT scanning table in the supine position with their knees flexed to permit the spine to be flattened in an effort to minimize the need for individual Gantry angles for each lumbar vertebrae; however, the fifth lumbar vertebra required a different Gantry angle for parallel sections. A restraint was placed around the knees to reduce motion artifact. The study was performed at 140 KV, 100 MA and targeted to include L-spine and retro-spinal muscle and fat. Vertebral bodies were acquired from L1 to L5, and included 4–5 sections through the pedicle level; the scan was performed over 2 s with an 18 cm field size and a slice thickness of 5 mm oriented parallel to the vertebral end plate. Scans were acquired by several radiology technicians but processed by a single radiology technician. A radiologist reviewed the films. Two sections were selected as the most representative of the medullary portion of each of the vertebral body (Figure 1). In the Picker Q-BMAP software, the technique uses the patient's muscle and fat tissue as internal reference standard as the X-ray attenuation properties of these soft tissues remains relatively constant.18 The Q-BMAP software uses the measured CT values for fat (−85.7 Hounsfield units (HU)) and muscle (27.1 HU), which permit definition of a reference line to allow the conversion of the CT values for trabecular bone into equivalent bone density (mg/cc). At this time, images were transferred from a Q-Series scanner to a Q-Series BMAP system with single image display in which the selected image of trabecular bone, ROI, was circumscribed by computerized localization for determination of BMD. The Q-BMAP software also provided a T-score, which was calculated by determining the deviation of the BMD from that of young normal values, divided by the standard deviation (s.d.) of the reference population. The most recent International Society for Clinical Densitometry guideline does not permit the clinical diagnosis of osteoporosis from T-scores derived from qCT as these values are not equivalent to the T-scores derived by DXA.19
Computed tomographic image of the lumbar spine at the level of the pedicle (arrow). The region of interest (ROI) is drawn within the trabecular lumbar vertebral body, excluding cortical bone and extraneous calcification, if present. Bone mineral density (BMD) is obtained by measuring the Hounsfield units in the ROI and comparing the value to units from soft tissue.19
The L-spine was evaluated by computerized tomographic scan for the degree of DJD. A semiquantitative technique was used to grade the severity of DJD because mild, moderate or severe. An experienced radiologist performed the grading of the L-spine of each subject.
Statistical analyses
The results are expressed as mean plus or minus s.d. of the mean. An unpaired T-score was used to determine the presence of significance between the group with SCI and the control group for L-spine BMD and T-scores by DXA and qCT. Although the T-scores derived from qCT are not equivalent to those derived by DXA, this does not preclude a relative comparison between values obtained from each method. A paired T-test was applied to determine significance between imaging methods for T-scores. A χ2 analysis was used to determine the significance of the distribution differences between groups for DJD. A two-factor (DJD, method) analysis of variance was applied for comparisons of T-scores, and Fisher-protected least squares differences were used for post hoc comparisons. Linear regression analysis was applied to determine the relationship between L-spine BMD and level of lesion (each level of lesion was assigned a number to correspond to vertebral segment from 1 (C1) to 25 (L4)).
Results
The groups were well-matched for age, height, weight and BMI (m/kg2) with no significant differences (Table 1). Persons with SCI had an average duration of injury of 14±11 years (range: 1–31 years) (Table 1). There was no significant difference between the SCI and control groups for L-spine DXA BMD (1.391±0.210 vs 1.315±0.178 g/m2; Figure 2a) or for L-spine DXA T-score (1.471±1.794 vs 0.782±1.481; Figure 2b). However, L-spine qCT BMD was significantly lower in the SCI compared with the control group (1.296±0.416 vs 1.572±0.382 g/m2, P=0.05; Figure 2a) and the T-score approached significance (−1.838±1.366 vs −0.963±1.227, P=0.059; Figure 2b). Six subjects with SCI had T-scores ⩽−2.5 s.d. below the mean by qCT, whereas all had T-scores >−2.5 s.d. below the mean by DXA (Figure 3). All control subjects had T-scores >−2.5 s.d. below the mean by DXA, and only one subject had T-scores ⩽−2.5 s.d. below the mean by qCT (Figure 3).
Mean values for bone mineral density (BMD) and T-scores of the lumbar spine (L-spine). The solid boxes represent the control group, and the lightly shaded boxes represent the spinal cord injury (SCI) group. (a) BMD of the L-spine by dual-energy X-ray absorptiometry (DXA) (1.315±0.178 vs 1.391±0.210 g/m2; not significant (NS)) and quantitative computerized tomography (qCT) (1.572±0.382 vs 1.296±0.416, *P=0.05) (b) T-scores of the L-spine by DXA (0.782±1.481 vs 1.471±1.794); NS) and qCT (−0.963±1.277 vs −1.838±1.366, P=0.059).
T-scores of the lumbar spine for quantitative computerized tomography (qCT) and dual-energy X-ray absorptiometry (DXA) by subject. Lines connect individual subjects. (a) Spinal cord injury (SCI) subjects are represented by the solid circles (•). All subjects with SCI had T-scores >−2.5 s.d. below the mean by DXA, and six SCI subjects had T-scores ⩽−2.5 s.d. by qCT. (b) The able-bodied controls are represented by the open circles (○). All control subjects had T-scores >−2.5 s.d. below the mean by DXA, and only one able-bodied subject had a T-scores ⩽−2.5 s.d. below the mean by qCT.
Of the 20 subjects with SCI, 11 had mild or moderate DJD and 5 had severe DJD, whereas only 6 of 15 control subjects had mild DJD (χ2(2)=7.84, P<0.02). The median T-score difference (DXA T-score minus qCT T-score) was significantly higher in those with SCI than that of controls (3.5±2.0 vs 1.7±1.3, P<0.01) (Figure 4). The effect of the severity of DJD on the T-score by DXA was evident when the groups were stratified by the extent of disease. Those with moderate to severe DJD, classifications only present in the SCI group in our limited study, had significantly higher T-scores by DXA than those without or with mild DJD, and significant differences for T-scores between DXA and qCT were noted for group by severity of DJD (Figure 5).
Box plots of the differences between lumbar spine T-scores (dual-energy X-ray absorptiometry T-score minus quantitative computerized tomography T-score). Each horizontal line in the plot of the controls and subjects with spinal cord injury (SCI) represents a percentile (from bottom to top: 10th, 25th, 50th, 75th and 90th percentile) for the T-score difference. The 25th to the 75th percentiles are represented by a cross-hatched box. There is a significant difference between the median values (middle horizontal line) for the SCI group and the control group (3.5±2.0 vs 1.7±1.3, *P<0.01). The solid circles (•) represent outliers.
Box plots of all subjects (spinal cord injury (SCI) and control) categorized by severity of lumbar spine degenerative joint disease (DJD). Each horizontal line in each plot represents a percentile (from bottom to top: 10th, 25th, 50th, 75th and 90th percentile) for the T-scores. The 25th to the 75th percentiles are represented by boxes with the following designations: open box=control subjects without or with mild DJD, lightly shaded box=SCI subjects without or with mild DJD and darkly shaded box=SCI subjects with moderate or severe DJD. *P<0.001 comparing T-scores for dual-energy X-ray absorptiometry (DXA) with quantitative computerized tomography (qCT) within subgroups categorized by degree of DJD (for example, mild, moderate and severe). ‡P<0.002 for T-scores differences among the subgroups (control subjects without or with mild DJD, SCI subjects without or with mild DJD and SCI subjects with moderate or severe DJD). The solid circles (•) represent outliers. Note that the subgroup with the most severe DJD had the highest T-score value by DXA.
A significant relationship was evident for L-spine BMD by qCT and level of lesion (r=0.46, P<0.05), with the higher cord lesions generally having lower L-spine BMD. No such relationship was shown for L-spine BMD by DXA and level of lesion. Of note, BMD of the L-spine of subjects with tetraplegia was significantly lower than that of able-bodied controls (1.128±0.310 vs 1.572±0.099, P<0.01), and this comparison approached significance for those with paraplegia (1.464±0.455 vs 1.572±0.099, P=0.069).
Discussion
There has been a lack of consensus and clinical confusion with regard to bone mass of the L-spine in persons with chronic SCI. Despite the presence of accepted risk factors for osteoporosis of advancing age, menopause and duration of immobilization, earlier studies employing standard DXA imaging techniques alone have consistently reported an absence of vertebral bone loss, or, in some reports, even increased veterbral bone mass.1, 14, 20 Our findings showed that DXA failed to identify osteopenia and osteoporosis of the L-spine in individuals with chronic SCI. If the L-spine is measured by qCT, men with chronic SCI have significant loss of spine bone mass, as they do of the extremities below the level of lesion.1, 3, 8, 9, 11 Generally, earlier studies that have relied on AP DXA imaging of the lumbar vertebrae may be presumed to have captured extraneous calcification of, and associated with, the spine, thus providing spurious findings of preservation of the bone mass.
In 1988, Biering–Sorensen reported that although bone mineral content of the femoral neck and shaft and proximal tibia were significantly reduced in persons with SCI, there seemed to be preservation of bone mass of the L-spine, which was postulated to be due to maintenance of weight on the spine while seated.8 Employing AP DXA as the methodology to acquire regional BMD, Leslie and Nance10 compared 14 men with chronic SCI with 46 men with various endocrine disorders that predispose to bone loss (for example, exogenous steroid administration, hypogonadism, hyperthyroidism and other predisposing conditions) and idiopathic osteoporosis.10 Although persons with SCI had marked femoral neck bone loss (86% of age-matched normal), these individuals had no evidence of L-spine loss (102% of age-matched normal). In contrast, the patients with endocrine disorders or idiopathic osteoporosis had a disproportionate demineralization of the spine (82% of age-matched normal) and minimal loss of BMD of the femoral neck (98% of age-matched normal). Using DXA as the imaging method, women with SCI after menopause have been reported to have an increase in L-spine BMD, in striking contrast to able-bodied post-menopausal women.11 Our findings in men with SCI raise the distinct possibility that in women with SCI the apparent increase of L-spine BMD measured by DXA may have been due to extraneous calcification.
Jaovisidha et al.12 performed a correlation analysis of vertebral levels L1–L4 between AP radiographs of the L-spine and DXA in 116 persons with chronic SCI. By DXA, elevation of BMD was observed at all lumbar levels, ranging from 15 to 20%; 49% of the vertebrae were abnormal on routine spine films. The authors concluded that the elevated DXA results for BMD were because of secondary progressive skeletal abnormalities that served to prevent the identification of significant bone loss. Liu et al.13 studied a cohort of 64 persons with chronic SCI by qCT of the L-spine, and they also investigated a subgroup of 29 of the 64 persons using both qCT and DXA. In the total group, the Z-scores derived by qCT were −2.0±1.2 of historic age-matched controls; in the subgroup in which both imaging techniques were performed, the qCT Z-score was −2.4±1.1 and for DXA, 1.3±2.3 above the mean, which is quite similar to the finding reported herein.13 Indeed, in the general population qCT seems to be a more sensitive technique for the diagnosis of osteopenia and to correlate better with fracture risk than that of DXA.21, 22 However, qCT is not the method of choice for monitoring serial changes in BMD owing to its inferior precision, relatively high radiation exposure, more difficult positioning, longer duration of scan and higher cost than DXA.23, 24
Of note, a correlation was shown between L-spine BMD by qCT and level of lesion. The subjects with higher levels of lesion generally had lower L-spine bone density. It may be speculated that higher lesions, especially if associated with greater motor impairment, would have greater degrees of impairment of the trunk musculature, and thus less forces associated with stress and strain due to muscular contraction on the vertebrae.
In an able-bodied population of 750 men and 750 women studied for moderate or severe deformities of the spine, 8% of men and 7% of women had a moderate deformity of the spine, whereas 4% of men and 8% of women had a severe deformity of the spine.25 In those with SCI, 55% (11 of the 20 subjects) had mild to moderate DJD and 25% (5 of the 20 subjects) had severe DJD of the L-spine. In contrast, in the control group, 40% (6 of the 15 subjects) had mild DJD and none had moderate or severe DJD of the L-spine. As such, both from our study and from a comparison with the general literature, it would appear that persons with SCI have an increased prevalence of abnormalities of the spine.12, 13 BMD measurements of the L-spine derived by qCT and DXA are acquired by different techniques and, in addition, are not measuring the same regions of interest. However, in the absence of confounding effects, one would have expected the T-scores to be somewhat comparable. Owing to the greater disparity of the L-spine T-scores between DXA and qCT for the group with SCI compared with the control group, it may have been expected that those with SCI would have had increased extraneous calcification in the imaging field. By categorizing the group with SCI by the severity of DJD, the subjects with the most extensive disease were also the ones who had spuriously higher lumbar T-scores by DXA.
The literature of skeletal bone mass in persons with SCI has largely relied on the accuracy of DXA to diagnose osteoporosis. Using DXA imaging to diagnose osteoporosis of the extremities in those with SCI is usually valid and useful. However, relying on AP DXA alone to determine BMD of the axial skeleton is problematic. Because of the apparent increased likelihood of osteophytes, vascular calcification, microcompression fractures and other skeletal abnormalities in persons with SCI, relying solely on AP DXA measurement of vertebral body density should be avoided. Quantitative CT or possibly, as suggested in the literature, lateral or midlateral DXA of the spine, if the technical capacity is available to perform this measurement, are the preferred methods of imaging of the L-spine in individuals with degenerative changes. An alternative approach before performing DXA imaging may be to obtain radiographs of the L-spine to exclude extraneous calcification. Otherwise, the clinician may be misled to believe that lumbar vertebral BMD is normal or even increased for age, and thus underestimate the risk of vertebral fracture.
Conclusion
In contrast to the majority of literature that has relied on diagnosing vertebral bone mass by DXA, this study has shown that a proportion of men with chronic SCI, especially those with higher cord lesions, have significant bone loss of the L-spine. A clear association has been shown between the presence and severity of extraneous spine calcification, and spuriously elevated L-spine BMD and T-scores. The use of qCT permits isolation and examination of the medullary portion of trabecular bone of the vertebral body, rather than relying on the AP projection by DXA that captures the posterior cortical bone and potentially associated pathological bony and vascular structures. Because persons with SCI may engage in physical activities to strengthen their upper bodies, significant loss of bone mass of the vertebrae would be expected to place them at a heightened risk of vertebral compression fracture. As such, it is prudent to consider avoiding exercises that place large compressive forces on the spine until a reliable imaging technique can be applied to exclude significant loss of bone mass of the L-spine.
References
Bauman WA, Spungen AM, Schwartz E, Wang J, Pierson Jr RN . Continuous loss of bone in chronic immobilization: a monozygotic twin study. Osteopor Int 1999; 10: 123–127.
Griffiths HJ, Zimmerman RE . The use of photon densitometry to evaluate bone mineral in a group of patients with spinal cord injury. Paraplegia 1973; 10: 279–284.
Garland DE, Stewart CA, Adkins RH, Hu SS, Rosen C, Liotta FJ et al. Osteoporosis after spinal cord injury. J Orthopaed Res 1992; 10: 279–284.
Comarr AE, Hutchinson RH, Bors E . Extremity fractures of patients with spinal cord injuries. Am J Surg 1962; 103: 732–739.
Freehafer A, Coletta M, Becker CL . Lower extremity fractures in patients with spinal cord injury. Paraplegia 1981; 19: 367–372.
Ragnarsson K, Sell G . Lower extremity fractures after spinal cord injury: a retrospective study. Arch Phys Med Rehabil 1981; 62: 418–423.
Szollar SM, Martin EM, Parthemore JG, Sartoris DJ, Deftos LJ . Demineralization in tetraplegic and paraplegic man over time. Spinal Cord 1997; 35: 223–238.
Biering-Sorensen F, Bohr H, Schaadt O . Bone mineral content of the lumbar spine and lower extremities years after spinal cord lesion. Paraplegia 1988; 26: 293–301.
Chow YW, Inman C, Pollintine P, Sharp CA, Haddaway MJ, el Masry W et al. Ultrasound bone densitometry and dual energy X-ray absorptiometry in patients with spinal cord injury: a cross-sectional study. Spinal Cord 1996; 34: 736–741.
Leslie WD, Nance PW . Dissociated hip and spine demineralization: a specific finding in spinal cord injury. Arch Phys Med Rehabil 1993; 74: 960–964.
Garland DE, Adkins RH, Stewart CA, Ashford R, Vigil D . Regional osteoporosis in women who have a complete spinal cord injury. J Bone Joint Surg Am 2001; 83: 1195–1200.
Jaovisidha S, Sartoris DJ, Martin EME, De Maeseneer M, Szollar SM, Deftos LJ . Influence of sponylopathy on bone densitometry using dual energy X-ray absorptiometry. Calcif Tissue Int 1997; 60: 424–429.
Liu CC, Theodorou DJ, Theodorou SJ, Andre MP, Sartoris DJ, Szollar SM et al. Quantitative computed tomography in the evaluation of spinal osteoporosis following spinal cord injury. Osteoporos Inter 2000; 11: 889–896.
Yu W, Gluer CC, Fuerst T, Grampp S, Li J, Lu Y et al. Influence of degenerative joint disease on spinal bone mineral measurements in postmenopausal women. Calcif Tissue Int 1995; 57: 169–174.
Finkelstein JS, Cleary RL, Butler JP, Antonelli R, Mitlak BH, Deraska DJ et al. A comparison of lateral versus anterior-posterior spine dual energy X-ray absorptiometry for the diagnosis of osteopenia. J Clin Endocrinol Metab 1994; 78: 724–730.
Reeve J, Abraham R, Walton J, Russell L, Wardley-Smith B, Mitchell A . Increasing mineral density after menopause in individual lumbar vertebrae as a marker for incident degenerative disease: a pilot study for the effects of body composition and diet. J Rheumatol 2004; 31: 1986–1992.
Pietrobelli A, Formica C, Wang Z, Heymsfield SB . Dual-energy X-ray absorptiometry body composition model: review of physical concepts. Am J Physiol 1996; 271: E941–E951.
Boden SD, Goodnough DJ, Stockham CD, Jacobs E . Precise measurement of vertebral bone density using computed tomography without the use of an external reference phantom. J of Digital Imaging 1989; 2: 31–38.
Engelke K, Adams JE, Armbrecht G, Augat P, Bogado CE, Bouxsein ML et al. Clinical use of quantitative computed tomography and peripheral quantitative computed tomography in the management of osteoporosis in adults—The 2007 ISCD Offical Postitions. J Clin Densitom 2008; 11: 123–162.
Riggs BL, Wahner HW, Seeman E, Offord KP, Dunn WL, Mazess RB et al. Changes in bone mineral density of the proximal femur and spine with aging. Differences between the postmenopausal and senile osteoporosis syndromes. J Clin Invest 1982; 70: 716–723.
Yu W, Gluer CC, Grampp S, Jergas M, Fuerst T, Wu CY et al. Spinal bone mineral assessment in postmenopausal women: a comparison between dual X-ray absorptiometry and quantitative computed tomography. Osteoporos Int 1995; 5: 433–439.
Pacifici R, Rupich R, Griffin M, Chines A, Susman N, Avioli LV . Dual energy radiography versus quantitative computer tomography for the diagnosis of osteoporosis. J Clin Endocrinol Metab 1990; 70: 705–710.
Rosenthal DI, Ganott MA, Wyshak G, Slovik DM, Doppelt SH, Neer RM . Quantitative computed tomography for spinal density measurement. Factors affecting precision. Invest Radiol 1985; 20: 306–310.
Johnson CCJ, Slemaenda CW, Melton III LJ . Clinical use of bone densitometry. N Engl J Med 1991; 324: 1105–1109.
Burger H, Van Daele PLA, Grashuis K, Hofman A, Grobbee DE, Schütte HE et al. Vertebral deformities and functional impairment in men and women. J Bone Miner Res 1997; 12: 152–257.
Acknowledgements
The authors wish to thank United Spinal Association (formally Eastern Paralyzed Veterans Association), The James J Peters Medical Center, Bronx, NY, the Department of Veterans Affairs Rehabilitation Research & Development Service, and the Kessler Institute for Rehabilitation, West Orange, NJ, for their support. This work was funded by a Rehabilitation Research & Development (RR&D) Service Merit Review entitled ‘Bone Loss in Acute and Chronic Spinal Cord Injury’ (#B98-1452) & RR&D Center of Excellence for the Medical Consequences of Spinal Cord Injury (#B2648C & B4162C).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bauman, W., Schwartz, E., Song, I. et al. Dual-energy X-ray absorptiometry overestimates bone mineral density of the lumbar spine in persons with spinal cord injury. Spinal Cord 47, 628–633 (2009). https://doi.org/10.1038/sc.2008.169
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2008.169
Keywords
This article is cited by
-
Bone Loss and the Current Diagnosis of Osteoporosis and Risk of Fragility Fracture in Persons with Spinal Cord Injury
Current Physical Medicine and Rehabilitation Reports (2020)
-
Osteoporosis in Veterans with Spinal Cord Injury: an Overview of Pathophysiology, Diagnosis, and Treatments
Clinical Reviews in Bone and Mineral Metabolism (2019)
-
Dual-energy X-ray absorptiometry and fracture prediction in patients with spinal cord injuries and disorders
Osteoporosis International (2017)
-
Risk factors for osteoporotic fractures in persons with spinal cord injuries and disorders
Osteoporosis International (2016)
-
A comprehensive study of long-term skeletal changes after spinal cord injury in adult rats
Bone Research (2015)