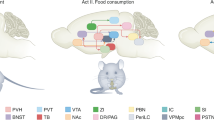
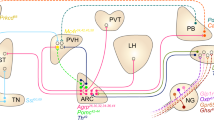
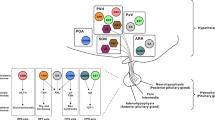
Abstract
Body weight and adiposity represent biologically controlled parameters that are influenced by a combination of genetic, developmental and environmental variables. Although the hypothalamus plays a crucial role in matching caloric intake with energy expenditure to achieve a stable body weight, it is now recognized that neuronal circuits in the hindbrain not only serve to produce nausea and to terminate feeding in response to food consumption or during pathological states, but also contribute to the long-term control of body weight. Additionally, recent work has identified hindbrain neurons that are capable of suppressing food intake without producing aversive responses like those associated with nausea. Here we review recent advances in our understanding of the hindbrain neurons that control feeding, particularly those located in the area postrema and the nucleus tractus solitarius. We frame this information in the context of new atlases of hindbrain neuronal populations and develop a model of the hindbrain circuits that control food intake and energy balance, suggesting important areas for additional research.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Data and Statistics on Overweight and Obesity. (Centers for Disease Control and Prevention, accessed 1 April 2022) https://www.cdc.gov/obesity/data/adult.html
Affinati, A. H. & Myers, M. G., Jr. in Endotext (eds Feingold, K. R. et al.) Neuroendocrine control of body energy homeostasis. (South Dartmouth, 2000).
Myers, M. G. Jr., Affinati, A. H., Richardson, N. & Schwartz, M. W. Central nervous system regulation of organismal energy and glucose homeostasis. Nat. Metab. 3, 737–750 (2021).
Myers, M. G. Jr. & Olson, D. P. SnapShot: neural pathways that control feeding. Cell Metab. 19, 732–732e1 (2014).
Berthoud, H. R., Munzberg, H., Richards, B. K. & Morrison, C. D. Neural and metabolic regulation of macronutrient intake and selection. Proc. Nutr. Soc. 71, 390–400 (2012).
Willer, C. J. et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat. Genet. 41, 25–34 (2009).
Saper, C. B., Chou, T. C. & Elmquist, J. K. The need to feed: homeostatic and hedonic control of eating. Neuron 36, 199–211 (2002).
Marks, D. L., Butler, A. A. & Cone, R. D. Melanocortin pathway: animal models of obesity and disease. Ann. Endocrinol. 63, 121–124 (2002).
Ring, L. E. & Zeltser, L. M. Disruption of hypothalamic leptin signaling in mice leads to early-onset obesity, but physiological adaptations in mature animals stabilize adiposity levels. J. Clin. Invest. 120, 2931–2941 (2010).
Grill, H. J. & Kaplan, J. M. The neuroanatomical axis for control of energy balance. Front. Neuroendocrinol. 23, 2–40 (2002).
Grill, H. J. & Hayes, M. R. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 16, 296–309 (2012).
Kim, M., Heo, G. & Kim, S. Y. Neural signalling of gut mechanosensation in ingestive and digestive processes. Nat. Rev. Neurosci. 23, 135–156 (2022).
Kaplan, J. M., Seeley, R. J. & Grill, H. J. Daily caloric intake in intact and chronic decerebrate rats. Behav. Neurosci. 107, 876–881 (1993).
D’Agostino, G. et al. Nucleus of the solitary tract serotonin 5-HT2C receptors modulate food intake. Cell Metab. 28, 619–630 (2018).
Macia, L. et al. Macrophage inhibitory cytokine 1 (MIC-1/GDF15) decreases food intake, body weight and improves glucose tolerance in mice on normal and obesogenic diets. PLoS ONE 7, e34868 (2012).
Breit, S. N., Tsai, V. W. & Brown, D. A. Targeting obesity and cachexia: identification of the GFRAL receptor-MIC-1/GDF15 pathway. Trends Mol. Med. 23, 1065–1067 (2017).
Coester, B. et al. RAMP1 and RAMP3 differentially control amylin’s effects on food intake, glucose and energy balance in male and female mice. Neuroscience 447, 74–93 (2020).
Turek, V. F. et al. Mechanisms of amylin/leptin synergy in rodent models. Endocrinology 151, 143–152 (2010).
Burmeister, M. A. et al. The hypothalamic glucagon-like peptide 1 receptor is sufficient but not necessary for the regulation of energy balance and glucose homeostasis in mice. Diabetes 66, 372–384 (2017).
Lutz, T. A., Mollet, A., Rushing, P. A., Riediger, T. & Scharrer, E. The anorectic effect of a chronic peripheral infusion of amylin is abolished in area postrema/nucleus of the solitary tract (AP/NTS) lesioned rats. Int. J. Obes. Relat. Metab. Disord. 25, 1005–1011 (2001).
Adams, J. M. et al. Liraglutide modulates appetite and body weight through glucagon-like peptide 1 receptor-expressing glutamatergic neurons. Diabetes 67, 1538–1548 (2018).
Hsu, J. Y. et al. Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature 550, 255–259 (2017).
Yang, L. et al. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat. Med. 23, 1158–1166 (2017).
Emmerson, P. J. et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat. Med. 23, 1215–1219 (2017).
Mullican, S. E. et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat. Med. 23, 1150–1157 (2017).
Worth, A. A. et al. The cytokine GDF15 signals through a population of brainstem cholecystokinin neurons to mediate anorectic signalling. eLife 9, e55164 (2020).
Cheng, W. et al. NTS Prlh overcomes orexigenic stimuli and ameliorates dietary and genetic forms of obesity. Nat. Commun. 12, 5175 (2021).
Cheng, W. et al. Calcitonin receptor neurons in the mouse nucleus tractus solitarius control energy balance via the non-aversive suppression of feeding. Cell Metab. 31, 301–312 (2020).
Kanoski, S. E., Rupprecht, L. E., Fortin, S. M., De Jonghe, B. C. & Hayes, M. R. The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology 62, 1916–1927 (2012).
Roman, C. W., Sloat, S. R. & Palmiter, R. D. A tale of two circuits: CCKNTS neuron stimulation controls appetite and induces opposing motivational states by projections to distinct brain regions. Neuroscience 358, 316–324 (2017).
Roman, C. W., Derkach, V. A. & Palmiter, R. D. Genetically and functionally defined NTS to PBN brain circuits mediating anorexia. Nat. Commun. 7, 11905 (2016).
D’Agostino, G. et al. Appetite controlled by a cholecystokinin nucleus of the solitary tract to hypothalamus neurocircuit. eLife 5, e12225 (2016).
Carter, M. E., Han, S. & Palmiter, R. D. Parabrachial calcitonin gene-related peptide neurons mediate conditioned taste aversion. J. Neurosci. 35, 4582–4586 (2015).
Campos, C. A., Bowen, A. J., Schwartz, M. W. & Palmiter, R. D. Parabrachial CGRP neurons control meal termination. Cell Metab. 23, 811–820 (2016).
Campos, C. A. et al. Cancer-induced anorexia and malaise are mediated by CGRP neurons in the parabrachial nucleus. Nat. Neurosci. 20, 934–942 (2017).
Palmiter, R. D. The parabrachial nucleus: CGRP neurons function as a general alarm. Trends Neurosci. 41, 280–293 (2018).
Campos, C. A., Bowen, A. J., Roman, C. W. & Palmiter, R. D. Encoding of danger by parabrachial CGRP neurons. Nature 555, 617–622 (2018).
Kim, D. Y. et al. A neural circuit mechanism for mechanosensory feedback control of ingestion. Nature 580, 376–380 (2020).
Tsang, A. H., Nuzzaci, D., Darwish, T., Samudrala, H. & Blouet, C. Nutrient sensing in the nucleus of the solitary tract mediates non-aversive suppression of feeding via inhibition of AgRP neurons. Mol. Metab. 42, 101070 (2020).
Beutler, L. R. et al. Dynamics of gut–brain communication underlying hunger. Neuron 96, 461–475 (2017).
Lawrence, C. B., Celsi, F., Brennand, J. & Luckman, S. M. Alternative role for prolactin-releasing peptide in the regulation of food intake. Nat. Neurosci. 3, 645–646 (2000).
Kim, S. H. et al. Pancreatic beta cell function following liraglutide-augmented weight loss in individuals with prediabetes: analysis of a randomised, placebo-controlled study. Diabetologia 57, 455–462 (2014).
Dodd, G. T. et al. The thermogenic effect of leptin is dependent on a distinct population of prolactin-releasing peptide neurons in the dorsomedial hypothalamus. Cell Metab. 20, 639–649 (2014).
Ludwig, M. Q. et al. A genetic map of the mouse dorsal vagal complex and its role in obesity. Nat. Metab. 3, 530–545 (2021).
Opitz, K. & Schafer, G. The effect of lithium on food intake in rats. Int. Pharmacopsychiatry 11, 197–205 (1976).
Gaykema, R. P. et al. Activation of murine pre-proglucagon-producing neurons reduces food intake and body weight. J. Clin. Invest. 127, 1031–1045 (2017).
Cheng, W. et al. Leptin receptor-expressing nucleus tractus solitarius neurons suppress food intake independently of GLP1 in mice. JCI Insight 5, e134359 (2020).
Richard, J. E. et al. Activation of the GLP-1 receptors in the nucleus of the solitary tract reduces food reward behavior and targets the mesolimbic system. PLoS ONE 10, e0119034 (2015).
Zhang, C. et al. Area postrema cell types that mediate nausea-associated behaviors. Neuron 109, 461–472 (2021).
Dowsett, G. K. C. et al. A survey of the mouse hindbrain in the fed and fasted states using single-nucleus RNA sequencing. Mol. Metab. 53, 101240 (2021).
Tao, J. et al. Highly selective brain-to-gut communication via genetically defined vagus neurons. Neuron 109, 2106–2115 (2021).
Aklan, I. et al. NTS catecholamine neurons mediate hypoglycemic hunger via medial hypothalamic feeding pathways. Cell Metab. 31, 313–326 (2020).
Chen, J. et al. A vagal–NTS neural pathway that stimulates feeding. Curr. Biol. 30, 3986–3998 (2020).
Resch, J. M. et al. Aldosterone-sensing neurons in the NTS exhibit state-dependent pacemaker activity and drive sodium appetite via synergy with angiotensin II signaling. Neuron 96, 190–206 (2017).
Do, J., Chang, Z., Sekerkova, G., McCrimmon, D. R. & Martina, M. A leptin-mediated neural mechanism linking breathing to metabolism. Cell Rep. 33, 108358 (2020).
Huo, L., Gamber, K. M., Grill, H. J. & Bjorbaek, C. Divergent leptin signaling in proglucagon neurons of the nucleus of the solitary tract in mice and rats. Endocrinology 149, 492–497 (2008).
Hayes, M. R. et al. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab. 11, 77–83 (2010).
Paul, S., Mondal, G. P., Bhattacharyya, R., Ghosh, K. C. & Bhat, I. A. Neuromyelitis optica spectrum disorders. J. Neurol. Sci. 420, 117225 (2021).
Mullican, S. E. & Rangwala, S. M. Uniting GDF15 and GFRAL: therapeutic opportunities in obesity and beyond. Trends Endocrinol. Metab. 29, 560–570 (2018).
Borner, T. et al. GDF15 induces an aversive visceral malaise state that drives anorexia and weight loss. Cell Rep. 31, 107543 (2020).
Borner, T. et al. GDF15 induces anorexia through nausea and emesis. Cell Metab. 31, 351–362 (2020).
Petry, C. J. et al. Associations of vomiting and antiemetic use in pregnancy with levels of circulating GDF15 early in the second trimester: a nested case–control study. Wellcome Open Res. 3, 123 (2018).
Sabatini, P. V. et al. GFRAL-expressing neurons suppress food intake via aversive pathways. Proc. Natl Acad. Sci. USA 118, e2021357118 (2021).
Ludwig, M. Q., Todorov, P. V., Egerod, K. L., Olson, D. P. & Pers, T. H. Single-cell mapping of GLP-1 and GIP receptor expression in the dorsal vagal complex. Diabetes 70, 1945–1955 (2021).
Lawrence, C. B., Ellacott, K. L. & Luckman, S. M. PRL-releasing peptide reduces food intake and may mediate satiety signaling. Endocrinology 143, 360–367 (2002).
Boyle, C. N., Lutz, T. A. & Le Foll, C. Amylin—its role in the homeostatic and hedonic control of eating and recent developments of amylin analogs to treat obesity. Mol. Metab. 8, 203–210 (2018).
Hayes, M. R. et al. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab. 13, 320–330 (2011).
Secher, A. et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J. Clin. Invest. 124, 4473–4488 (2014).
Fortin, S. M., Chartoff, E. H. & Roitman, M. F. The aversive agent lithium chloride suppresses phasic dopamine release through central GLP-1 receptors. Neuropsychopharmacology 41, 906–915 (2016).
Drucker, D. J. The biology of incretin hormones. Cell Metab. 3, 153–165 (2006).
Borner, T. et al. GIP receptor agonism attenuates GLP-1 receptor agonist-induced nausea and emesis in preclinical models. Diabetes 70, 2545–2553 (2021).
Frias, J. P. et al. Efficacy and tolerability of tirzepatide, a dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist in patients with type 2 diabetes: a 12-week, randomized, double-blind, placebo-controlled study to evaluate different dose-escalation regimens. Diabetes Obes. Metab. 22, 938–946 (2020).
Baggio, L. L. & Drucker, D. J. Glucagon-like peptide-1 receptor co-agonists for treating metabolic disease. Mol. Metab. 46, 101090 (2021).
Campbell, J. E. Targeting the GIPR for obesity: to agonize or antagonize? potential mechanisms. Mol. Metab. 46, 101139 (2021).
Zhang, Q. et al. The glucose-dependent insulinotropic polypeptide (GIP) regulates body weight and food intake via CNS–GIPR signaling. Cell Metab. 33, 833–44 (2021).
Boer, G. A., Keenan, S. N., Miotto, P. M., Holst, J. J. & Watt, M. J. GIP receptor deletion in mice confers resistance to high-fat diet-induced obesity via alterations in energy expenditure and adipose tissue lipid metabolism. Am. J. Physiol. Endocrinol. Metab. 320, E835–E845 (2021).
Williams, E. K. et al. Sensory neurons that detect stretch and nutrients in the digestive system. Cell 166, 209–221 (2016).
Bai, L. et al. Genetic identification of vagal sensory neurons that control feeding. Cell 179, 1129–1143 (2019).
Han, W. et al. A neural circuit for gut-induced reward. Cell 175, 665–678 (2018).
Jin, H., Fishman, Z. H., Ye, M., Wang, L. & Zuker, C. S. Top-down control of sweet and bitter taste in the mammalian brain. Cell 184, 257–271 (2021).
Zhang, J. et al. Sour sensing from the tongue to the brain. Cell 179, 392–402 (2019).
Biddinger, J. E., Lazarenko, R. M., Scott, M. M. & Simerly, R. Leptin suppresses development of GLP-1 inputs to the paraventricular nucleus of the hypothalamus. eLife 9, e59857 (2020).
Acknowledgements
The Banbury conference on Integrated Control of Feeding and Energy Balance by Hypothalamic and Hindbrain Circuits stimulated much of the thinking underlying this article and related work, and we thank R. Leshan, L. Heisler and the attendees of the conference for sharing their thoughts. We also thank C. Rhodes, D. Sandoval and D. Olson and members of their laboratories, along with members of the laboratories of R.J.S., T.H.P. and M.G.M. for helpful discussions. Supported by AstraZeneca, National Institutes of Health (grant P01 DK117821), the American Diabetes Association (1-16-PDF-021 to W.C.), the Lundbeck Foundation (grant no. R190-2014-3904 to T.H.P.), the Novo Nordisk Foundation (grant no. NNF18CC0034900 to the Novo Nordisk Foundation Center for Basic Metabolic Research, which is an independent research centre, based at the University of Copenhagen), and the Danish Diabetes Academy, which is funded by the Novo Nordisk Foundation (grant no. NNF17SA0031406).
Author information
Authors and Affiliations
Contributions
M.G.M., W.C. and R.J.S. wrote the first draft of the manuscript; all the other authors extensively edited and revised the manuscript. M.G.M. drafted the figures, and W.C. revised the figures in line with comments from the other authors. M.G.M. is the guarantor of the paper.
Corresponding author
Ethics declarations
Competing interests
M.G.M., T.H.P. and R.J.S. receive research support from Novo Nordisk, and M.G.M. and R.J.S. receive research support from AstraZeneca. M.G.M. is a paid consultant for AstraZeneca and Regeneron Pharmaceuticals. R.J.S. also receives research support from Fractyl and is a paid consultant for Novo Nordisk, Scohia, ShouTi Pharma and Fractyl. R.J.S. also has equity positions in Calibrate and Rewind. The other authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Zachary Knight and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Ashley Castellanos-Jankiewicz, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cheng, W., Gordian, D., Ludwig, M.Q. et al. Hindbrain circuits in the control of eating behaviour and energy balance. Nat Metab 4, 826–835 (2022). https://doi.org/10.1038/s42255-022-00606-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-022-00606-9
This article is cited by
-
Artesunate treats obesity in male mice and non-human primates through GDF15/GFRAL signalling axis
Nature Communications (2024)
-
Reciprocal activity of AgRP and POMC neurons governs coordinated control of feeding and metabolism
Nature Metabolism (2024)
-
Paracrine signalling by pancreatic δ cells determines the glycaemic set point in mice
Nature Metabolism (2024)
-
Adrenergic modulation of melanocortin pathway by hunger signals
Nature Communications (2023)
-
Sequential appetite suppression by oral and visceral feedback to the brainstem
Nature (2023)