Abstract
It has been suggested that, in states of arousal, release of noradrenaline and β-adrenergic signalling affect long-term memory formation by stimulating astrocytic lactate production from glycogen. However, the temporal relationship between cortical activity and cellular lactate fluctuations upon changes in arousal remains to be fully established. Also, the role of β-adrenergic signalling and brain glycogen metabolism on neural lactate dynamics in vivo is still unknown. Here, we show that an arousal-induced increase in cortical activity triggers lactate release into the extracellular space, and this correlates with a fast and prominent lactate dip in astrocytes. The immediate drop in astrocytic lactate concentration and the parallel increase in extracellular lactate levels underline an activity-dependent lactate release from astrocytes. Moreover, when β-adrenergic signalling is blocked or the brain is depleted of glycogen, the arousal-evoked cellular lactate surges are significantly reduced. We provide in vivo evidence that cortical activation upon arousal triggers lactate release from astrocytes, a rise in intracellular lactate levels mediated by β-adrenergic signalling and the mobilization of lactate from glycogen stores.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data that support the findings of this study are available from the corresponding author upon request. Source Data for Extended Data Fig. 8 are available online.
Code availability
All code that was used in this study is available from the corresponding author upon request. The CHIPS toolbox for MATLAB is freely available on GitHub (https://ein-lab.github.io/)37.
References
Kety, S. S. & Schmidt, C. F. The nitrous oxide method for the quantitative determination of cerebral blood flow in man: theory, procedure and normal values. J. Clin. Invest. 27, 476–483 (1948).
Hu, Y. & Wilson, G. S. A temporary local energy pool coupled to neuronal activity: fluctuations of extracellular lactate levels in rat brain monitored with rapid-response enzyme-based sensor. J. Neurochem. 69, 1484–1490 (1997).
Newman, L. A., Korol, D. L. & Gold, P. E. Lactate produced by glycogenolysis in astrocytes regulates memory processing. PLoS ONE 6, e28427 (2011).
Yellen, G. Fueling thought: management of glycolysis and oxidative phosphorylation in neuronal metabolism. J. Cell Biol. 217, 2235–2246 (2018).
Magistretti, P. J. & Allaman, I. Lactate in the brain: from metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 19, 235–249 (2018).
Dienel, G. A. Lack of appropriate stoichiometry: strong evidence against an energetically important astrocyte-neuron lactate shuttle in brain. J. Neurosci. Res. 95, 2103–2125 (2017).
Barros, L. F. & Weber, B. CrossTalk proposal: an important astrocyte-to-neuron lactate shuttle couples neuronal activity to glucose utilisation in the brain. J. Physiol. 596, 347–350 (2018).
Pellerin, L. & Magistretti, P. J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc. Natl Acad. Sci. USA 91, 10625–10629 (1994).
Wyss, M. T., Jolivet, R., Buck, A., Magistretti, P. J. & Weber, B. In vivo evidence for lactate as a neuronal energy source. J. Neurosci. 31, 7477–7485 (2011).
Machler, P. et al. In vivo evidence for a lactate gradient from astrocytes to neurons. Cell Metab. 23, 94–102 (2016).
Diaz-Garcia, C. M. et al. Neuronal stimulation triggers neuronal glycolysis and not lactate uptake. Cell Metab. 26, 361–374 e364 (2017).
Suzuki, A. et al. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144, 810–823 (2011).
Zhang, Y. et al. Inhibition of lactate transport erases drug memory and prevents drug relapse. Biol. Psychiatry 79, 928–939 (2016).
Duran, J., Saez, I., Gruart, A., Guinovart, J. J. & Delgado-Garcia, J. M. Impairment in long-term memory formation and learning-dependent synaptic plasticity in mice lacking glycogen synthase in the brain. J. Cereb. Blood Flow Metab. 33, 550–556 (2013).
Gao, V. et al. Astrocytic β2-adrenergic receptors mediate hippocampal long-term memory consolidation. Proc. Natl Acad. Sci. USA 113, 8526–8531 (2016).
Descalzi, G., Gao, V., Steinman, M. Q., Suzuki, A. & Alberini, C. M. Lactate from astrocytes fuels learning-induced mRNA translation in excitatory and inhibitory neurons. Commun. Biol. 2, 247 (2019).
Paukert, M. et al. Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron 82, 1263–1270 (2014).
Alberini, C. M., Cruz, E., Descalzi, G., Bessieres, B. & Gao, V. Astrocyte glycogen and lactate: new insights into learning and memory mechanisms. Glia 66, 1244–1262 (2018).
Dienel, G. A. & Cruz, N. F. Aerobic glycolysis during brain activation: adrenergic regulation and influence of norepinephrine on astrocytic metabolism. J. Neurochem. 138, 14–52 (2016).
Sorg, O. & Magistretti, P. J. Characterization of the glycogenolysis elicited by vasoactive intestinal peptide, noradrenaline and adenosine in primary cultures of mouse cerebral cortical astrocytes. Brain Res. 563, 227–233 (1991).
Sada, N., Lee, S., Katsu, T., Otsuki, T. & Inoue, T. Epilepsy treatment. Targeting LDH enzymes with a stiripentol analog to treat epilepsy. Science 347, 1362–1367 (2015).
Clasadonte, J., Scemes, E., Wang, Z., Boison, D. & Haydon, P. G. Connexin 43-mediated astroglial metabolic networks contribute to the regulation of the sleep-wake cycle. Neuron 95, 1365–1380 (2017).
Tang, F. et al. Lactate-mediated glia-neuronal signalling in the mammalian brain. Nat. Commun. 5, 3284 (2014).
Morland, C. et al. The lactate receptor, G-protein-coupled receptor 81/hydroxycarboxylic acid receptor 1: expression and action in brain. JJ. Neurosci. Res. 93, 1045–1055 (2015).
San Martin, A. et al. A genetically encoded FRET lactate sensor and its use to detect the Warburg effect in single cancer cells. PLoS ONE 8, e57712 (2013).
Chen, T. W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Ohkura, M., Sasaki, T., Kobayashi, C., Ikegaya, Y. & Nakai, J. An improved genetically encoded red fluorescent Ca2+ indicator for detecting optically evoked action potentials. PLoS ONE 7, e39933 (2012).
Franks, N. P. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat. Rev. Neurosci. 9, 370–386 (2008).
Liu, X. et al. Arousal transitions in sleep, wakefulness, and anesthesia are characterized by an orderly sequence of cortical events. NeuroImage 116, 222–231 (2015).
Kum, J. E., Han, H. B. & Choi, J. H. Pupil size in relation to cortical states during isoflurane anesthesia. Exp. Neurobiol. 25, 86–92 (2016).
Guedel, A. E. Inhalation Anesthesia; A Fundamental Guide (Macmillan, 1951).
Chen, C. R. et al. Roles of adrenergic alpha1 and dopamine D1 and D2 receptors in the mediation of the desynchronization effects of modafinil in a mouse EEG synchronization model. PLoS ONE 8, e76102 (2013).
McCormick, D. A. & Bal, T. Sleep and arousal: thalamocortical mechanisms. Annu. Rev. Neurosci. 20, 185–215 (1997).
Jameson, L. C. & Sloan, T. B. Using EEG to monitor anesthesia drug effects during surgery. J. Clin. Monit. Comput. 20, 445–472 (2006).
MacIver, M. B. & Bland, B. H. Chaos analysis of EEG during isoflurane-induced loss of righting in rats. Front. Syst. Neurosci. 8, 203 (2014).
Reimer, J. et al. Pupil fluctuations track fast switching of cortical states during quiet wakefulness. Neuron 84, 355–362 (2014).
Barrett, M. J. P., Ferrari, K. D., Stobart, J. L., Holub, M. & Weber, B. CHIPS: an extensible toolbox for cellular and hemodynamic two-photon image analysis. Neuroinformatics 16, 145–147 (2018).
Stobart, J. L. et al. Cortical circuit activity evokes rapid astrocyte calcium signals on a similar timescale to neurons. Neuron 98, 726–735 e724 (2018).
Gibbs, M. E., Anderson, D. G. & Hertz, L. Inhibition of glycogenolysis in astrocytes interrupts memory consolidation in young chickens. Glia 54, 214–222 (2006).
Lee, K., Makino, S., Imagawa, T., Kim, M. & Uehara, M. Effects of adrenergic agonists on glycogenolysis in primary cultures of glycogen body cells and telencephalon astrocytes of the chick. Poult. Sci. 80, 1736–1742 (2001).
Duran, J. & Guinovart, J. J. Brain glycogen in health and disease. Mol. Asp. Med. 46, 70–77 (2015).
Berridge, C. W. & Foote, S. L. Effects of locus coeruleus activation on electroencephalographic activity in neocortex and hippocampus. J. Neurosci. 11, 3135–3145 (1991).
Carter, M. E. et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat. Neurosci. 13, 1526–1533 (2010).
Aston-Jones, G. & Cohen, J. D. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 28, 403–450 (2005).
Berridge, C. W. & Waterhouse, B. D. The locus coeruleus–noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res. Brain Res. Rev. 42, 33–84 (2003).
Ding, F. et al. α1-Adrenergic receptors mediate coordinated Ca2+ signaling of cortical astrocytes in awake, behaving mice. Cell Calcium 54, 387–394 (2013).
Srinivasan, R. et al. Ca2+ signaling in astrocytes from Ip3r2–/– mice in brain slices and during startle responses in vivo. Nat. Neurosci. 18, 708–717 (2015).
Slezak, M. et al. Distinct mechanisms for visual and motor-related astrocyte responses in mouse visual cortex. Curr. Biol. 29, 3120–3127 (2019).
Zerbi, V. et al. Rapid reconfiguration of the functional connectome after chemogenetic locus coeruleus activation. Neuron 103, 702–718 (2019).
Chaigneau, E., Oheim, M., Audinat, E. & Charpak, S. Two-photon imaging of capillary blood flow in olfactory bulb glomeruli. Proc. Natl Acad. Sci. USA 100, 13081–13086 (2003).
Sotelo-Hitschfeld, T. et al. Channel-mediated lactate release by K+-stimulated astrocytes. J. Neurosci. 35, 4168–4178 (2015).
Ruminot, I., Schmalzle, J., Leyton, B., Barros, L. F. & Deitmer, J. W. Tight coupling of astrocyte energy metabolism to synaptic activity revealed by genetically encoded FRET nanosensors in hippocampal tissue. J. Cereb. Blood Flow Metab. 39, 513–523 (2019).
Pierre, K. & Pellerin, L. Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J. Neurochem. 94, 1–14 (2005).
Karagiannis, A. et al. Hemichannel-mediated release of lactate. J. Cereb. Blood Flow Metab. 36, 1202–1211 (2016).
Hung, Y. P., Albeck, J. G., Tantama, M. & Yellen, G. Imaging cytosolic NADH-NAD+ redox state with a genetically encoded fluorescent biosensor. Cell Metab. 14, 545–554 (2011).
Kasparov, S. Are astrocytes the pressure-reservoirs of lactate in the brain? Cell Metab. 23, 1–2 (2016).
Lerchundi, R. et al. NH4+ triggers the release of astrocytic lactate via mitochondrial pyruvate shunting. Proc. Natl Acad. Sci. USA 112, 11090–11095 (2015).
Bolanos, J. P., Delgado-Esteban, M., Herrero-Mendez, A., Fernandez-Fernandez, S. & Almeida, A. Regulation of glycolysis and pentose-phosphate pathway by nitric oxide: impact on neuronal survival. Biochim. Biophys. Acta 1777, 789–793 (2008).
Choi, H. B. et al. Metabolic communication between astrocytes and neurons via bicarbonate-responsive soluble adenylyl cyclase. Neuron 75, 1094–1104 (2012).
Schwarz, L. A. & Luo, L. Organization of the locus coeruleus-norepinephrine system. Curr. Biol. 25, R1051–R1056 (2015).
Cahill, L., Prins, B., Weber, M. & McGaugh, J. L. β-adrenergic activation and memory for emotional events. Nature 371, 702–704 (1994).
Murchison, C. F. et al. A distinct role for norepinephrine in memory retrieval. Cell 117, 131–143 (2004).
Sun, H., Mao, Y., Wang, J. & Ma, Y. Effects of β-adrenergic antagonist, propranolol on spatial memory and exploratory behavior in mice. Neurosci. Lett. 498, 133–137 (2011).
Bittner, C. X. et al. Fast and reversible stimulation of astrocytic glycolysis by K+ and a delayed and persistent effect of glutamate. J. Neurosci. 31, 4709–4713 (2011).
Ruminot, I. et al. NBCe1 mediates the acute stimulation of astrocytic glycolysis by extracellular K+. J. Neurosci. 31, 14264–14271 (2011).
Savaki, H. E., Kadekaro, M., Jehle, J. & Sokoloff, L. α- and β-adrenoreceptor blockers have opposite effects on energy metabolism of the central auditory system. Nature 276, 521–523 (1978).
Catus, S. L., Gibbs, M. E., Sato, M., Summers, R. J. & Hutchinson, D. S. Role of β-adrenoceptors in glucose uptake in astrocytes using β-adrenoceptor knockout mice. Br. J. Pharmacol. 162, 1700–1715 (2011).
Dong, J. H. et al. β2-adrenergic receptor and astrocyte glucose metabolism. J. Mol. Neurosci. 48, 456–463 (2012).
Ma, Z., Stork, T., Bergles, D. E. & Freeman, M. R. Neuromodulators signal through astrocytes to alter neural circuit activity and behaviour. Nature 539, 428–432 (2016).
Mayrhofer, J. M., Haiss, F., Helmchen, F. & Weber, B. Sparse, reliable, and long-term stable representation of periodic whisker deflections in the mouse barrel cortex. NeuroImage 115, 52–63 (2015).
Mayrhofer, J. M. et al. Novel two-alternative forced choice paradigm for bilateral vibrotactile whisker frequency discrimination in head-fixed mice and rats. J. Neurophysiol. 109, 273–284 (2013).
Mayrhofer, J. M. et al. Design and performance of an ultra-flexible two-photon microscope for in vivo research. Biomed. Opt. Express 6, 4228–4237 (2015).
Pologruto, T. A., Sabatini, B. L. & Svoboda, K. ScanImage: flexible software for operating laser scanning microscopes. Biomed. Eng. Online 2, 13 (2003).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Coggan, J. S. et al. Norepinephrine stimulates glycogenolysis in astrocytes to fuel neurons with lactate. PLoS Comput. Biol. 14, e1006392 (2018).
Acknowledgements
We thank all Weber group laboratory members for support and frequent discussions. We would also like to thank J.-C. Paterna and the Viral Vector Facility of the Neuroscience Center Zurich (ZNZ) for virus production. We further thank J. M. Mayrhofer and W. v. d. Behrens for their initial support in establishing the suppression-response task. A.S.S. received support from a Synapsis Career Fellowship Award and the Cloëtta Foundation, M.T.W. is supported by the Hartmann-Müller Foundation, L.F.B. is funded by the Chilean Government through the Centers of Excellence Basal Financing Program of CONICYT, J.D. and J.J.G. are funded by the Spanish MINECO (BFU2017-82375-R) and the National Institutes of Health (NIH-NINDS) (P01NS097197) and B.W. is supported by the Swiss National Science Foundation (SNF number:31003A_156965).
Author information
Authors and Affiliations
Contributions
Conceptualization, M.Z., A.S.S., M.T.W. and B.W.; Methodology, M.Z.; Software, M.Z. and K.D.F.; Formal analysis, M.Z. and A.S.S.; Investigations, M.Z., Z.J.L. and L.H.; Resources: J.L.S., J.D., J.J.G. and L.F.B.; Writing—original draft, M.Z. and A.S.S.; Visualization, M.Z. and A.S.S.; Writing—review and editing, M.Z., A.S.S., M.T.W., L.F.B. and B.W.; Supervision and project administration, M.T.W. and B.W.; Funding acquisition, M.Z., A.S.S., M.T.W. and B.W. All authors discussed the results and contributed to revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Primary Handling Editor: Elena Bellafante.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Awake two-photon imaging to study cortical lactate and calcium dynamics in neurons and astrocytes (Related to Fig. 1).
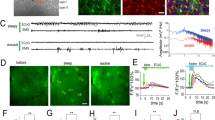
(a) Workflow scheme of preparing mice for awake two-photon imaging. Following recovery from surgery and intracortical AAV injections, mice were trained for head-restrained imaging using a suppression-response task and a water reward. On average, mice were ready for imaging at around 4 weeks after surgery. This protocol was repeated for all animals in this study with similar results. (b) Behavioral paradigm used during awake imaging. Each trial consisted of an 8 s imaging period (IM) which ended with a 1 ms auditory cue (AC) indicating the mouse to collect the water reward within a lick period of 2 s (LP). Each trial had an intertrial interval (ITI) of 2 s before the next trial started. (c) Mean behavioral performance (grey) and learning progress (black) during training. A trial was counted correct when no licking occurred during image acquisition (IM). N = number of animals, n = number of sessions. (d) Cortical expression of genetically-encoded lactate sensor Laconic (San Martin et al., 25), calcium sensor RCaMP 1.07 (Ohkura et al., 27) and GCaMP6s (Chen et al., 26,32) imaged through a chronic cranial window. Visualization with single photon excitation. M = medial, L = lateral, C = cranial, R = rostral. (e) Representative images of Laconic expression in neurons and astrocytes (mTFP = blue; Venus = yellow), RCaMP 1.07 in neurons (mApple = red) and GCaMP6s in astrocytes (cpGFP = green) and a merge of calcium sensors expressed at the same site (neurons = red, astrocytes = green). Excitation wavelength for Laconic = 870 nm. Wavelength for simultaneous excitation of GCaMP6s and RCaMP 1.07 = 940 nm. Images were taken 121.4 ± 26.9 µm below cortical surface.
Extended Data Fig. 2 Lactate dynamics in cortical neurons and astrocytes upon prolonged isoflurane exposure (Related to Fig. 1).
(a) Scheme of the prolonged isoflurane imaging protocol. T indicates time points in minutes throughout the imaging session. Time points for the onset (T10) and end (T30) of isoflurane delivery are indicated by START and STOP. (b, c) Cortical neurons (b, left panel) and astrocytes expressing Laconic (c, left panel) in individual fields of view. Single cell fluorescence analysis of relative lactate changes (middle panels) during the prolonged isoflurane administration protocol. Both, neurons and astrocytes showed a rise in lactate levels that persisted during the isoflurane period (indicated at T30; relative lactate signal rose by 4.6 ± 1.6% in neurons and 5.5 ± 2.0 % in astrocytes; p < 0.001). At T10.2 upon isoflurane induction relative lactate signal dropped by -1.8 ± 1.1 % (p < 0.007) in astrocytes only. In contrast, neurons showed no significant lactate decrease when compared to baseline (p > 0.98). Lactate levels return to baseline during recovery (indicated at T60). Bar graphs summarize relative changes at T5, T10.2, T30 and T60. Data set contained 104 neurons and 102 astrocytes. T indicates time points in minutes. N = number of animals, n = number of experiments. Data is represented as mean ± SD. m = 6 experiments used to derive statistics. Mean statistics were calculated using two-tailed linear mixed-effects models and Tukey’s post hoc tests.
Extended Data Fig. 3 Isoflurane exposure causes an initial arousal response before reaching the anesthetized state (Related to Fig. 1).
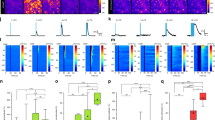
(a) EEG raw traces at different time points of the prolonged isoflurane imaging protocol (Extended Data Fig. 2a). Traces represent distinct states such as: T5 = awake behaving, T10.2 = induction and desynchronization, T30 = anesthetized and increased slow-wave activity leading to burst suppression, T60 = recovery and awake. (b) EEG power spectrum and EEG bands (delta: 0.5-4 Hz, theta: 4-8 Hz, alpha: 8-12 Hz, beta: 12-30 Hz and gamma: 30-50 Hz) show a clear shift upon isoflurane exposure. Blue = low power (30 dB) and red = high power (90 dB). (c) EEG bands according to their power display a biphasic EEG response when transiting from the awake state through the induction phase (increase in high-frequency power) to the anesthetized state (increase in low-frequency power). (d) Quantification of EEG bands as a relative change with respect to baseline at time points T5, T10.2, T30 and T60 derived from (c). (e) EMG spectrum (10-30 Hz) recorded from neck muscles showed an increase in muscle activity with exposure to isoflurane and a reduced power in the anesthetized state. Blue = low power (30 dB) and red = high power (90 dB). (f) Pupillometry analysis revealed pupil dilation in the induction phase (T10.2), then a pupil constriction in the anesthetized state (T30). White circles indicate pupil size approximation. (g) Relative changes in EMG power (black) and pupil radius (blue) recorded during the isoflurane protocol. (h) Lick performance during the isoflurane protocol. Note that prolonged isoflurane administration immobilized the animal and behavioral performance is absent. (i) Quantification of EMG and pupil radius and lick performance at time points T5, T10.2, T30 and T60. Note a significant increase in neuromuscular activity in the induction phase (T10.2) and a reduction in EMG power, pupil size and lick performance during the anesthetized state highlighting that prolonged isoflurane exposure first initiates an aroused state (within the first 60 s), which is then gradually followed by an anesthetized state and immobilization. T indicates time points in minutes. N = number of animals, n = number of experiments. START and STOP indicate the period of isoflurane administration. Data is represented as mean ± SD. m = 10 experiments for EEG, 8 for EMG, 6 for pupil and 8 for lick performance used to derive statistics. Mean statistics were calculated using two-tailed linear mixed-effects models and Tukey’s post hoc tests.
Extended Data Fig. 4 Calcium activity in neurons and astrocytes upon isoflurane administration (Related to Fig. 1).
(a) Scheme of the prolonged isoflurane imaging protocol consisting of a 10 min baseline (awake behaving), 20 min isoflurane and a 30 min recovery period. T indicates time in min for the course of the imaging session. Time points for the onset (T10) and end (T30) of isoflurane delivery are indicated by START and STOP. (b, c) Whole frame fluorescence analysis of relative calcium changes during the isoflurane administration protocol (a). The onset of isoflurane exposure (START) caused a strong activation in cortical network and a significant rise in neuronal and astrocytic calcium levels (T10.2). In neurons, whole frame fluorescence intensity increased by 14.2 ± 7.3% (p < 0.001) and in astrocytes, it increased by 50.1 ± 21.2 % (p < 0.001). During the anesthetized state (T30) overall calcium levels decreased in both cell types and recovered to baseline levels when mice returned to the awake state (T60). Bar graphs on the right summarize calcium level changes at time points T5, T10.2, T30 and T60. (d, e) Automated analysis to identify calcium events for neurons (d) and astrocytes (e) using MATLAB toolbox CHIPS (Barrett et al., 37). Detected signals are indicated by colored ROI masks for time points T5, T10.2, T30 and T60 of the isoflurane administration protocol (a). ROIs represent cellular areas of calcium events detected by an automated activity-based algorithm and do not reflect specific manually selected cell structures. Quantification of the number of events detected during these time points are summarized in bar graphs (right). With isoflurane administration there was a significant increase in calcium events in both neurons and astrocytes (T10.2) compared to baseline (T5). Event frequencies were strongly reduced during the anesthetized state (T30) and recovered back to baseline at T60. Example traces illustrate automatically detected events at T5, T10.2, T30 and T60 for neurons (d) and astrocytes (e). ROI = region of interest. T indicates time points in minutes. N = number of animals, n = number of experiments. Data is represented as mean ± SD. m = 6 experiments used to derive statistics. Mean statistics were calculated using two-tailed linear mixed-effects models and Tukey’s post hoc tests.
Extended Data Fig. 5 Comparison of different stimulation paradigms (Related to Fig. 1).
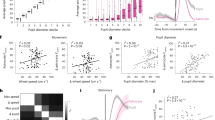
(a) Different paradigms to induce arousal in awake mice. (b) Comparison of pupil response strengths at T5.2 between the different paradigms. Whisker stimulation induced the smallest response (left) compared to a brief isoflurane pulse (strongest response; right). (c, d) Quantification of the area under the curve (AUC) of the stimulation evoked lactate dip in astrocytes (c) and the induced lactate surges in neurons (d; left) and astrocytes (d; right). Note that isoflurane pulse evoked the largest response when compared to other stimulation paradigms. (e) Comparison of stimulation paradigms and percentage of responding astrocytes and neurons. Only the ISO pulse mobilized the entire astrocytic and neuronal network. The threshold for a lactate dip (T5.2) or surge (T7) detection was set to 2 SD from baseline values (T2). (f) Quantification of lactate dips and surges of only responding neurons and astrocytes identified in (e). (g) Dose-dependent stimulation with isoflurane (air pulse, 0.75 % isoflurane and 1.5% isoflurane at 400 ml/min through a ventilation mask). (h) Quantification of evoked calcium events in astrocytes and neurons. Note that 1.5% isoflurane evoked the highest number of events. The same field of view was monitored for each paradigm. (i, j) The AUC for the dip in astrocytes and the surge in both cell types. (k) Percentages of astrocytes and neurons with a lactate response. (l) Quantification of the AUC of responding neurons and astrocytes only. (m) Time-dependent stimulation with isoflurane. (n) Comparison of response strengths measured by pupil response. (o, p) Quantification of lactate dips in astrocytes (o), and lactate surges in neurons and astrocytes (p). Note that the 20 s isoflurane pulse evoked a stronger response in neurons and astrocytes when compared to the 3 s stimulation paradigm. (q) Comparison of 3 s and 20 s isoflurane pulse stimulation regarding percentages of responding cells. (r) Quantification of the AUC of responding neurons and astrocytes only. The threshold for a lactate dip (T5.2) or surge (T7) detection was set to 2 SD from baseline values (T2). The same 120 neurons and 104 astrocytes were monitored for each paradigm. T indicates time points in minutes. N = number of animals, n = number of experiments. Data is represented as mean ± SD. m = 120 neurons and 104 astrocytes for lactate measurements or 6 experiments for calcium and pupil measurements used to derive statistics. Mean statistics were calculated using two-tailed linear mixed-effects models and Tukey’s post hoc tests.
Extended Data Fig. 6 Propranolol has no effect on cellular calcium activity but reduces lactate surge in astrocytes and neurons (Related to Fig. 3).
(a) Protocol used to investigate the effects of propranolol in neurons and astrocytes. Propranolol (10mg per kg, i.p.) was injected after baseline acquisition (0 h) and the same cells were imaged in subsequent sessions 1, 3, 6 and 24 h after injection. (b–e) ISO pulse-evoked calcium changes in neurons (b, c) and astrocytes (d, e) at different time points. Signal amplitudes did not differ from control experiments with saline injections (Extended Data Fig. 7). m = 6 experiments used for statistical analysis. (f, g) Temporal comparison of neuronal calcium and astrocytic lactate response before (0 h; black) and 6 h after (red) propranolol injection. Propranolol had no effect on neuronal calcium response (f) and the initial dip in astrocytic lactate upon arousal but it reduced the second phase of lactate mobilization by reducing the astrocytic lactate surge (g). Only means are displayed. (h) The fraction of astrocytes showing a dip and the fraction of astrocytes and neurons showing an activity-dependent surge. (i) The area under the curve (AUC) in responding astrocytes and the AUC of the surge of responding astrocytes and neurons. The threshold for a lactate dip (T5.2) or surge (T7) detection was set to 2 SD from baseline values (T2). The same 113 neurons and 118 were monitored for each paradigm. N = number of animals, n = number of experiments. Data is represented as mean ± SD. m = 113 neurons and 118 astrocytes for lactate measurements used to derive statistics. Mean statistics were calculated using two-tailed linear mixed-effects models and Tukey’s post hoc tests.
Extended Data Fig. 7 Control saline experiments of calcium and lactate responses in neurons and astrocytes (Related to Fig. 3).
(a) Scheme of the control protocol with saline for arousal-induced calcium and lactate responses in neurons and astrocytes. The same cells as in the propranolol paradigm were monitored in sequential imaging sessions. (b–e) Evoked calcium responses in neurons (b, c) and astrocytes (d, e). Calcium responses were not affected by saline injection. (f–i) Evoked lactate response in neurons (f) and astrocytes (h) before and after saline injection (0 h - 24 h). The same 113 neurons and 118 astrocytes were monitored in 6 experiments per time point. The isoflurane-induced lactate response in neurons (g) and astrocytes (i) was not affected by saline injection. Data is represented as mean ± SD. N = number of animals, n = number of experiments. Data was binned into 12 s fragments for data visibility in (b), (d), (f) and (h). m = 6 experiments used to derive statistics. Mean statistics were calculated two-tailed using linear mixed-effects models and Tukey’s post hoc tests.
Extended Data Fig. 8 Comparison of control animals and GYS KO mice (Related to Fig. 4).
(a) Immunohistological analysis of GYS1 KO (bottom) and control animals (litter mates; top) of cortical glycogen synthase 1 (GYS1; red) and glutamine synthetase (green). The merge illustrates loss of GYS1 in GYS1 KO mice. Graph represents quantification of the fluorescence intensity of GYS1 (17.2 ± 24.2 %) normalized to the mean of all control animals (100.0 ± 48.2 %; p < 0.001). (b) Western blot analysis of GYS1 protein abundance in GYS1 KO and control cortical tissue. Actin was used as loading control. Experiment was repeated 3 times with similar results. (c) Blood plasma glucose (left panel) and lactate (right panel) did not differ between GYS1 KO and control animals. (d) Automatically detected calcium events in cortical neurons (left) and astrocytes (right) in GYS1 KO mice (red) and littermate control animals (black). Both have a similar resting state activity. (e, f) One-point calibration with trans-acceleration (3 min; 500 mM pyruvate; i.v.) did not differ between GYS1 KO and control mice. Data set for control animals comprised 110 neurons and 102 astrocytes. Data set for knock-out animals comprised 108 neurons and 87 astrocytes. N = number of animals, n = number of experiments. Data is represented as mean ± SD. m = number of experiments as stated in the representing figure used to derive statistics. Mean statistics were calculated using two-tailed linear mixed-effects models and Tukey’s post hoc tests.
Extended Data Fig. 9 Comparison of lactate levels in neurons and astrocytes (Related to Fig. 4).
(a) Trans-acceleration with pyruvate through a tail-vein catheter for one-point calibration of relative intracellular lactate levels in neurons and astrocytes in awake mice. A bolus injection of pyruvate (3 min; 500 mM; i.v.) artificially increased blood pyruvate levels that entered the brain through monocarboxylate transporters (MCT). Sudden increase in extracellular pyruvate (blue; top right) led to a rapid extrusion and decrease in intracellular lactate (red; top right). This process is termed trans-acceleration. MCT = monocarboxylate transporter. (b, c) A bolus injection of pyruvate resulted in a significant decrease in astrocytic (c) but not neuronal lactate levels (b) indicating significantly higher lactate levels in astrocytes. (d) Saline bolus injections had no effect on intracellular lactate levels. For trans-acceleration and saline experiments, the same cells were imaged. m = 6 experiments used for statistical analysis. (e) Trans-acceleration increased blood plasma lactate levels (e; right panel) leading to an overshoot of lactate in neurons and astrocytes (b, c). Glucose plasma levels remained stable (e; left panel). (f) Saline had neither an effect on blood plasma glucose nor lactate levels. (g, h) Comparison of the temporal kinetics of the ISO pulse (red) and trans-acceleration (black) protocol illustrating significant differences in kinetics. N = number of animals, n = number of experiments. Data is represented as mean ± SD. For the comparison 226 neurons and 236 astrocytes for the ISO pulse, and 230 neurons and 200 astrocytes for the trans-acceleration paradigm were used to derive statistics. Mean statistics were calculated using two-tailed linear mixed-effects models and Tukey’s post hoc tests.
Extended Data Fig. 10 Loss of brain glycogen leads to impaired lactate surges and associative learning in GYS1 KO mice (Related to Fig. 4).
(a) Comparison of responsive cells of control (black) and GYS1 KO mice (red). (b) Quantification of the area under the curve (AUC) of responding neurons and astrocytes for the dip and surge identified in a. Astrocytes and neurons with a surge had a lower AUC in GYS1 KO animals. For statistical analysis 177 neurons and 146 astrocytes were used. (c) GYS1 KO mice (red) showed impaired learning performance in the suppression‐response task when compared to control animals (black) resulting in more errors per trial. (d, e) Both groups reduced the response latency to the auditory cue, however, GYS1 KO mice responded significantly slower than the control (d) although GYS1 KO were similarly responsive (e). N = number of animals, n = number of experiments. Data is represented as mean ± SD. m = 30 sessions used to derive statistics. Mean statistics were calculated using two-tailed linear mixed-effects models and Tukey’s post hoc tests.
Supplementary information
Supplementary Information
Supplementary Figures 1 and 2
Source data
Source Data Extended Data Fig. 8
Unprocessed western blot image. Boxes indicate regions corresponding to the Extended Data Figure 8b
Rights and permissions
About this article
Cite this article
Zuend, M., Saab, A.S., Wyss, M.T. et al. Arousal-induced cortical activity triggers lactate release from astrocytes. Nat Metab 2, 179–191 (2020). https://doi.org/10.1038/s42255-020-0170-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-020-0170-4
This article is cited by
-
Oligodendrocyte–axon metabolic coupling is mediated by extracellular K+ and maintains axonal health
Nature Neuroscience (2024)
-
A conceptual framework for astrocyte function
Nature Neuroscience (2023)
-
Lactate biosensors for spectrally and spatially multiplexed fluorescence imaging
Nature Communications (2023)
-
Astrocytes amplify neurovascular coupling to sustained activation of neocortex in awake mice
Nature Communications (2022)
-
A high-throughput multiparameter screen for accelerated development and optimization of soluble genetically encoded fluorescent biosensors
Nature Communications (2022)