Abstract
Catecholamines stimulate the first step of lipolysis through protein kinase A (PKA)-dependent release of the lipid-droplet-associated protein abhydrolase domain containing 5 (ABHD5) from perilipin to coactivate the lipase adipose triglyceride lipase (ATGL). Here, we unmask a proteolytic and cardioprotective function of ABHD5. ABHD5 acts in vivo and in vitro as a serine protease that cleaves histone deacetylase 4 (HDAC4). Through the production of an amino-terminal polypeptide of HDAC4 (HDAC4-NT), ABHD5 inhibits MEF2-dependent gene expression and thereby controls glucose handling. ABHD5 deficiency leads to neutral-lipid storage disease in mice. Cardiac-specific gene therapy using the gene encoding HDAC4-NT does not protect against intracardiomyocyte lipid accumulation, but strikingly protects against heart failure, thereby challenging the concept of lipotoxicity-induced heart failure. ABHD5 levels are reduced in failing human hearts, and murine transgenic ABHD5 expression protects against pressure-overload-induced heart failure. These findings represent a conceptual advance by connecting lipid with glucose metabolism through HDAC4 proteolysis, and enable new translational approaches to treating cardiometabolic disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Raw data from experiments presented in Fig. 3f,g, Supplementary Table 1 and Extended Data Fig. 3d were deposited in the gene-expression omnibus (GEO), accession number GSE135662. Source Data are available for Figs. 1–4 and Extended Data Figs. 1–6, including all uncut gels of the immunoblotting figures in the manuscript, and all data supporting the findings of the manuscript can be obtained from the corresponding author upon request.
References
Lohse, M. J., Engelhardt, S. & Eschenhagen, T. What is the role of beta-adrenergic signaling in heart failure? Circ. Res. 93, 896–906 (2003).
Dewenter, M., von der Lieth, A., Katus, H. A. & Backs, J. Calcium signaling and transcriptional regulation in cardiomyocytes. Circ. Res. 121, 1000–1020 (2017).
Rockman, H. A., Koch, W. J. & Lefkowitz, R. J. Cardiac function in genetically engineered mice with altered adrenergic receptor signaling. Am. J. Physiol. 272, H1553–H1559 (1997).
Wang, H. et al. Unique regulation of adipose triglyceride lipase (ATGL) by perilipin 5, a lipid droplet-associated protein. J. Biol. Chem. 286, 15707–15715 (2011).
Yamaguchi, T. et al. CGI-58 facilitates lipolysis on lipid droplets but is not involved in the vesiculation of lipid droplets caused by hormonal stimulation. J. Lipid Res. 48, 1078–1089 (2007).
Sahu-Osen, A. et al. CGI-58/ABHD5 is phosphorylated on Ser239 by protein kinase a: control of subcellular localization. J. Lipid Res. 56, 109–121 (2015).
Haemmerle, G. et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat. Med. 17, 1076–1085 (2011).
Zierler, K. A. et al. Functional cardiac lipolysis in mice critically depends on comparative gene identification-58. J. Biol. Chem. 288, 9892–9904 (2013).
Pollak, N. M. et al. The interplay of protein kinase A and perilipin 5 regulates cardiac lipolysis. J. Biol. Chem. 290, 1295–1306 (2015).
Lass, A. et al. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in chanarin-dorfman syndrome. Cell Metab. 3, 309–319 (2006).
Cerk, I. K., Wechselberger, L. & Oberer, M. Adipose triglyceride lipase regulation: an overview. Curr. Protein Pept. Sci. 19, 221–233 (2018).
Yamaguchi, T. Crucial role of CGI-58/alpha/beta hydrolase domain-containing protein 5 in lipid metabolism. Biol. Pharm. Bull. 33, 342–345 (2010).
Chanarin, I. et al. Neutral-lipid storage disease: a new disorder of lipid metabolism. BMJ 1, 553–555 (1975).
Lefevre, C. et al. Mutations in CGI-58, the gene encoding a new protein of the esterase/lipase/thioesterase subfamily, in chanarin-dorfman syndrome. Am. J. Hum. Genet. 69, 1002–1012 (2001).
Radner, F. P. et al. Growth retardation, impaired triacylglycerol catabolism, hepatic steatosis, and lethal skin barrier defect in mice lacking comparative gene identification-58 (CGI-58). J. Biol. Chem. 285, 7300–7311 (2010).
Wang, W. et al. Sustained β1-adrenergic stimulation modulates cardiac contractility by Ca2+/calmodulin kinase signaling pathway. Circ. Res. 95, 798–806 (2004).
Fischer, T. H. et al. Ca2+/calmodulin-dependent protein kinase II and protein kinase A differentially regulate sarcoplasmic reticulum Ca2+ leak in human cardiac pathology. Circulation 128, 970–981 (2013).
Backs, J., Song, K., Bezprozvannaya, S., Chang, S. & Olson, E. N. CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J. Clin. Invest. 116, 1853–1864 (2006).
Backs, J. et al. Selective repression of MEF2 activity by PKA-dependent proteolysis of HDAC4. J. Cell Biol. 195, 403–415 (2011).
Lehmann, L. H. et al. A proteolytic fragment of histone deacetylase 4 protects the heart from failure by regulating the hexosamine biosynthetic pathway. Nat. Med. 24, 62–72 (2018).
Kronlage, M. et al. O-GlcNAcylation of histone deacetylase 4 protects the diabetic heart from failure. Circulation 140, 580–594 (2019).
Kim, Y. et al. The MEF2D transcription factor mediates stress-dependent cardiac remodeling in mice. J. Clin. Invest. 118, 124–132 (2008).
Yang, L. K. & Tao, Y. X. Physiology and pathophysiology of the β3-adrenergic receptor. Prog. Mol. Biol. Transl. Sci. 161, 91–112 (2019).
Wei, J. et al. Reversal of pathological cardiac hypertrophy via the MEF2-coregulator interface. JCI Insight 2, pii: 91068 (2017).
Pouleur, A. C. et al. Rationale and design of a multicentre, randomized, placebo-controlled trial of mirabegron, a β3-adrenergic receptor agonist on left ventricular mass and diastolic function in patients with structural heart disease β3-left ventricular hypertrophy (β3-LVH). ESC Heart Fail. 5, 830–841 (2018).
Wang, Y. et al. Adipocyte liver kinase b1 suppresses beige adipocyte renaissance through class iia histone deacetylase 4. Diabetes 66, 2952–2963 (2017).
Sohal, D. S. et al. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ. Res. 89, 20–25 (2001).
deAlmeida, A. C., van Oort, R. J. & Wehrens, X. H. Transverse aortic constriction in mice. J. Vis. Exp. 38, e1729 (2010).
Kreusser, M. M. et al. Cardiac CaM kinase II genes delta and gamma contribute to adverse remodeling but redundantly inhibit calcineurin-induced myocardial hypertrophy. Circulation 130, 1262–1273 (2014).
Muller, O. J., Schinkel, S., Kleinschmidt, J. A., Katus, H. A. & Bekeredjian, R. Augmentation of AAV-mediated cardiac gene transfer after systemic administration in adult rats. Gene Ther. 15, 1558–1565 (2008).
Geisler, A. et al. microRNA122-regulated transgene expression increases specificity of cardiac gene transfer upon intravenous delivery of AAV9 vectors. Gene Ther. 18, 199–209 (2011).
Jungmann, A., Leuchs, B., Katus, H. A., Rommelaere, J. & Muller, O. J. Protocol for efficient generation and characterization of adeno-associated viral (AAV) vectors. Hum. Gene Ther. Methods 28, 235–246 (2017).
Lehmann, L. H. et al. Essential role of sympathetic endothelin a receptors for adverse cardiac remodeling. Proc. Natl Acad. Sci. USA 111, 13499–13504 (2014).
Adams, J. et al. 13-cis retinoic acid inhibits development and progression of chronic allograft nephropathy. Am. J. Pathol. 167, 285–298 (2005).
Benkert, P., Biasini, M. & Schwede, T. Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 27, 343–350 (2011).
Bienert, S. et al. The SWISS-MODEL repository-new features and functionality. Nucleic Acids Res. 45, D313–D319 (2017).
Waterhouse, A. et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 46, W296–W303 (2018).
Guex, N., Peitsch, M. C. & Schwede, T. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: a historical perspective. Electrophoresis 30 (Suppl. 1), S162–S173 (2009).
Boeszoermenyi, A. et al. Structure of a CGI-58 motif provides the molecular basis of lipid droplet anchoring. J. Biol. Chem. 290, 26361–26372 (2015).
Badin, P. M. et al. Regulation of skeletal muscle lipolysis and oxidative metabolism by the co-lipase CGI-58. J. Lipid Res. 53, 839–848 (2012).
Poschl, J. M. et al. Effects of dietary supplementation of saturated fatty acids and of n-6 or n-3 polyunsaturated fatty acids on plasma and red blood cell membrane phospholipids and deformability in weanling guinea pigs. Lipids 34, 467–473 (1999).
Dodt, M., Roehr, J. T., Ahmed, R. & Dieterich, C. FLEXBAR-flexible barcode and adapter processing for next-generation sequencing platforms. Biology (Basel) 1, 895–905 (2012).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Trapnell, C. et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and cufflinks. Nat. Protoc. 7, 562–578 (2012).
Alexa, A., Rahnenfuhrer, J. & Lengauer, T. Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics 22, 1600–1607 (2006).
Acknowledgements
We thank M. Hagenmüller for help with figures and manuscript editing; Q. Sun, M. Oestringer, J. Krebs, U. Oehl and J. Hartmann for technical help; R. Zahn, K. Perez, K. Remans and J. Baßler for help with purification of recombinant HDAC4 and ABHD5; and S. Kaden for the help with electron microscopy. L.H.L. is recipient of the Clinician-Scientist Program (CSP) of the German Cardiac Society (DGK). M.O. is supported by the Austrian Science Fund (FWF): F73 SFB Lipid Hydrolysis. J.B. was supported by grants from the Deutsche Forschungsgemeinschaft (BA 2258/2-1BA; BA 2258/9-1; SFB 1118), the European Commission (FP7-Health-2010; MEDIA-261409) and the DZHK (Deutsches Zentrum für Herz-Kreislauf-Forschung—German Centre for Cardiovascular Research) and by the BMBF (German Ministry of Education and Research).
Author information
Authors and Affiliations
Contributions
J.B. and Z.H.J. designed the study. Z.H.J., S.K.D., J.T., M.D., C.X., F.S., D.S., X.G., L.W. B.C.W., G.F., and S.W.S. carried out experiments. Z.H.J., L.H.L., S.W.S., C.D, J.-H.G. and J.B. analysed and interpreted data. T.F, O.J.M, S.S., P.M., C.D, C.M., M.O., G.H, H.A.K., J.T., and J.B. provided research support and conceptual advice. Z.H.J. J.T. and J.B. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
Z.H.J., L.H.L, O.J.M, H.A.K. and J.B. filed a patent on HDAC4-NT and ABHD5 gene therapy (US9914912B2). All other authors declare no competing interests.
Additional information
Peer review information Primary Handling Editor: Christoph Schmitt.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
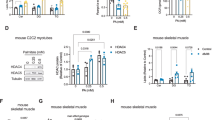
Extended Data Fig. 1 ABHD5 is required for HDAC4 proteolysis.
a, Immunoblotting using an antibody directed against endogenous ABHD5 in cardiac and cellular COS as well as HEK293 extracts. GAPDH was used as loading control. b, Cytosolic and nuclear fractions from adult rat ventricular myocytes were used for immunoblotting using an antibody directed against ABHD5, GAPDH and histone H3. a,b, Representative results of two independent experiments. c, Left, Immunoblotting analysis of cardiac extracts of control (Ctrl)- or isoproterenol (ISO)-treated mice using antibodies directed against HDAC4, ABHD5 and GAPDH as loading control. Middle left, Densitometric analysis of HDAC4-NT/FL ratio shown as fold change versus control. Middle right and right, Quantification of cardiac ABHD5 protein normalized to GAPDH and Abhd5 RNA levels normalized to 18S in unstressed mice and ISO-treated mice. Statistical analysis: Values are presented as mean ± s.e.m., n = 3 (n represents mice per indicated group); by unpaired two tailed t-test, P<0.05 considered as significant. d, Left, Immunoblotting analysis of cardiac extracts of control (Ctrl) or CL 316,243 (CL)-treated mice using antibodies directed against HDAC4, ABHD5 and GAPDH as loading control. Middle left, Densitometric analysis of HDAC4-NT/FL ratio shown as fold change versus control. Middle and Middle Right, Quantification of cardiac ABHD5 protein normalized to GAPDH and Abhd5 RNA levels normalized to 18S in unstressed mice and CL-treated mice. Right, Quantification of Ucp1 mRNA normalized to 18S in brown adipose tissue after treatment with CL as a marker of CL effective administration. Statistical analysis: Values are presented as mean ± s.e.m., n = 3 (n represents mice per indicated group); by unpaired two tailed t-test, P < 0.05 considered as significant.
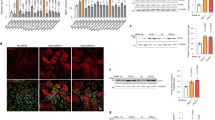
Extended Data Fig. 2 ABHD5 possesses intrinsic serine-protease activity.
a, Prediction of 3D structure of ABHD5 using SWISS-MODEL. Highlighted are aa predicted to serve as putative catalytic triad (Asn153, Ser298 and His327) and the putative phosphorylation site Ser-237. Ser-237 and Ser-298 of ABHD5 lie in spatial proximity to each other. Lys 51 represents the N-terminal starting aa of the peptide sequence used for modelling. Pink, cap domain; green, α/β-hydrolase core. b, Multiple sequence alignment of ABHD5 protein of different species as indicated by UniProt online tool (https://uniprot.org/align/). Red boxes denote aa N153, S298 and H327 of the predicted serine-protease catalytic triad, green box denotes aa S237 as reported PKA phosphorylation site (aa numbering corresponds to human ABHD5 protein). c, Tryptophan fluorescence decay plot upon thermal denaturation of rec-ABHD5-WT, -N153D, -S237A, -S237E -S298A and -H327A (1 °C min–1). Left, The ratio of fluorescence at 350 nm/330 nm was plotted against the temperature. Right, Equivalent analysis to identify the melting temperature (Tm) of the rec-proteins. Measurements were performed once. d, Coomassie staining after protease assay using recombinant purified HDAC4 (rec-HDAC4) and increasing amounts of recombinant purified ABHD5 (rec-ABHD5) as indicated. e, Coomassie staining after protease assay using rec-HDAC4 and rec-ABHD5 in the presence or absence of serine-protease-inhibitor AEBSF as indicated. f, Coomassie staining after protease assay using rec-HDAC4 and rec-ABHD5 WT or putative catalytic-triad mutants as indicated. The data in d–f represent results of three independent experiments. g, Immunoblotting and Coomassie staining for HDAC4 and ABHD5 after in vitro proteolysis assays involving rec-HDAC4, rec-ABHD5 WT or Ser-237 phospho-death (S237A) and phospho-mimetic (S237E) mutants as indicated. Coomassie staining was used to quantify the proteolytic activity of ABHD5. Statistical analysis: Values are presented as mean ± s.e.m., n = 3 (n represents independent experiments); by one-way ANOVA, P < 0.05 considered as significant. h, Immunoblotting for HDAC4 and ABHD5 in yeast-cell extracts after proteolysis assay in a yeast expression system as indicated. Because HDAC4 contains the Protein-A (PRA; 15 kDa) tag, HDAC4-NT is expected to migrate at 43 kDa. The experiment was performed at least three times with similar results.
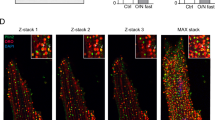
Extended Data Fig. 3 HDAC4-NT protects from ABHD5-deficiency induced cardiac dysfunction.
Cre-expressing control mice (Cre), cardiac-specific ABHD5-KO (cKO) and cKO with HDAC4-NT gene delivery (cKO+AAV9-NT) were analysed. a, Heart weight/body weight ratios (HW/BW) and fractional shortening (FS) are shown for the indicated groups. Statistical analysis: Values are presented as mean ± s.e.m.; n = 5 (n represents mice per indicated group); by one-way ANOVA, P < 0.05 considered as significant. b, Representative images of Methylene Blue staining of cardiac thin-sections to qualitatively visualize lipid droplet content in the indicated groups. Arrows point to positive lipid stain. c, Representative electron-microscopic images of the mitochondrial and sarcomeric structures of cardiomyocytes of control mice (Cre) and cKO mice; arrows point to the lipid accumulation on the mitochondrial and sarcomeric surface in cKO. b,c, Representative samples of one experiment with 5 mice included. d, RNA-seq analysis of heart tissue of the three above mentioned groups. The left panel shows a heat map displaying expression differences in cKO versus cKO+AAV9-NT. Right, Fold change in expression of selected genes in cKO versus Cre, cKO versus cKO+AAV9-NT and cKO+AAV9-NT versus Cre.
Extended Data Fig. 4 ABHD5 inhibits MEF2 and cardiomyocyte hypertrophy.
a, NRVMs were transduced with the Ad3×MEF2C-Luc reporter. Ad.FLAG–HDAC4, Ad.ABHD5 plus GFP on a bicistronic promoter and Ad.GFP (10 MOI) were then coexpressed as indicated, and NRVMs were stimulated for 24 h with ET-1 or FCS; n = 5 (n represents independent samples) and values are presented as mean ± s.e.m. b, Right panel: Fluorescence microscopy of NRVMs expressing Ad.ABHD5 plus GFP on a bicistronic promoter or Ad.GFP (10 MOI each). NRVM hypertrophy was induced with 100 nM ET-1 or 10% FCS for 24 h. Shown are representative images of α-actinin staining (red) of NRVMs and GFP fluorescence to confirm adenoviral expression. Images are representative of at least three independent experiments. Scale bar, 50 μm. Right panel, Quantification of NRVM cross-sectional areas (CSA). Statistical analysis: Values are presented as mean ± s.e.m., n = 4 (n represents independent samples); by one-way ANOVA, P < 0.05 considered as significant.
Extended Data Fig. 5 Generation and baseline characterization of cardiac-specific ABHD5 transgenic mice (TG).
a, Microinjected fragment of an expression plasmid containing hABHD5 cDNA flanked by α-MHC promoter and human growth hormone (hGH) poly-A (+) signal. b, Cardiac overexpression of ABHD5 in mice of 4 different transgenic lines as compared to wild-type littermates (WT) from the same lines was evaluated by immunoblotting analysis using an anti-ABHD5 antibody. c–e, Baseline characterization of transgenic mice. c, Representative echocardiography of a mouse from transgenic line #1 as compared to a WT littermate. The data in b are representative of 4 different mouse lines and the data in c are representative of 4 mice from line # 1 as well as 7 (WT) and 10 (TG) mice from line #9. d,e, Quantification of ejection fraction (EF), heart weight/body weight ratio (HW/BW), heart weight/tibia length ratio (HW/TL) in WT littermates (n = 4) and transgenic mice from line #1 (n = 4) (d) and in WT littermates (n = 7) and transgenic mice from line #9 (n = 10, TG) (e). d,e, Statistical analysis: Values are presented as mean ± s.e.m.; by unpaired two tailed t-test, P < 0.05 considered as significant. n represents mice per indicated group.
Extended Data Fig. 6 ABHD5 attenuates cardiac hypertrophy upon TAC.
a, Left panel: Quantification of HW/BW ratio of WT and TG mice three weeks after TAC or sham surgery. Right panel: Quantification of fractional shortening (FS) of WT and TG mice 3 weeks after TAC or sham surgery. Statistical analysis: Values are presented as mean ± s.e.m., n = 5 (n represents mice per indicated group); by one-way ANOVA, P < 0.05 considered as significant. b, mRNA expression of Abhd5 in sham and TAC-operated WT mice normalized to Gapdh. Statistical analysis: Values are presented as mean ± s.e.m., n = 4 (n represents mice per indicated group); by unpaired two tailed t-test, P < 0.05 are considered as significant. c, Corresponding band intensities of experiment shown in Fig. 4d were quantified. Left, Ratio between HDAC4-NT and HDAC4-FL. Middle, HDAC4 P-632 levels were determined upon TAC in relation to sham in WT and TG mice and normalized to GAPDH. Right, Ratio between HDAC4 P-632 and total HDAC4. Statistical analysis: Values are presented as mean ± s.e.m., n = 3 (n represents mice per indicated group); by unpaired two tailed t-test; P < 0.05 are considered as significant.
Supplementary information
Supplementary Information
Supplementary Tables 1–7
Supplementary Data 1
Human serine-protease siRNA library. List of siRNAs targeting known and predicted serine proteases (library was purchased from Ambion)
Supplementary Data 2
Prediction of the 3D structure of ABHD5 extracted from the online protein-structure-homology-modelling server SWISS-MODEL (https://swissmodel.expasy.org)
Source data
Source data Fig. 1
Unprocessed Western Blots and/or gels
Source Data Fig. 1
Statistical Source Data
Source data Fig. 2
Unprocessed Western Blots and/or gels
Source data Fig. 2
Statistical Source Data
Source data Fig. 3
Unprocessed Western Blots and/or gels
Source data Fig. 3
Statistical Source Data
Source data Fig. 4
Unprocessed Western Blots and/or gels
Source data Fig. 4
Statistical Source Data
Source data Extended Data Fig. 1
Unprocessed Western Blots and/or gels
Source data Extended Data Fig. 1
Statistical Source Data
Source data Extended Data Fig. 2
Unprocessed Western Blots and/or gels
Source data Extended Data Fig. 2
Statistical Source Data
Source data Extended Data Fig. 3
Statistical Source Data
Source data Extended Data Fig. 4
Statistical Source Data
Source data Extended Data Fig. 5
Unprocessed Western Blots and/or gels
Source data Extended Data Fig. 5
Statistical Source Data
Source data Extended Data Fig. 6
Statistical Source Data
Rights and permissions
About this article
Cite this article
Jebessa, Z.H., Shanmukha, K.D., Dewenter, M. et al. The lipid-droplet-associated protein ABHD5 protects the heart through proteolysis of HDAC4. Nat Metab 1, 1157–1167 (2019). https://doi.org/10.1038/s42255-019-0138-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-019-0138-4
This article is cited by
-
Lipolysis: cellular mechanisms for lipid mobilization from fat stores
Nature Metabolism (2021)
-
Cardiac-specific CGI-58 deficiency activates the ER stress pathway to promote heart failure in mice
Cell Death & Disease (2021)
-
Protein acetylation: a novel modus of obesity regulation
Journal of Molecular Medicine (2021)
-
Western diet induces severe nonalcoholic steatohepatitis, ductular reaction, and hepatic fibrosis in liver CGI-58 knockout mice
Scientific Reports (2020)
-
Therapeutic effects of histone deacetylase inhibitors on heart disease
Archives of Pharmacal Research (2020)