Abstract
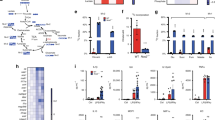
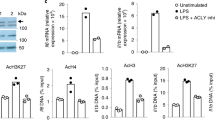
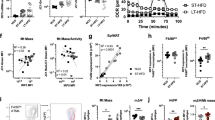
In response to signals associated with infection or tissue damage, macrophages undergo a series of dynamic phenotypic changes. Here we show that during the response to lipopolysaccharide and interferon-γ stimulation, metabolic reprogramming in macrophages is also highly dynamic. Specifically, the tricarboxylic acid cycle undergoes a two-stage remodelling: the early stage is characterized by a transient accumulation of intermediates including succinate and itaconate, whereas the late stage is marked by the subsidence of these metabolites. The metabolic transition into the late stage is largely driven by the inhibition of the pyruvate dehydrogenase complex (PDHC) and the oxoglutarate dehydrogenase complex (OGDC), which is controlled by the dynamic changes in the lipoylation state of both PDHC and OGDC E2 subunits and phosphorylation of the PDHC E1 subunit. This dynamic metabolic reprogramming results in a transient metabolic state that strongly favours hypoxia-inducible factor-1α (HIF-1α) stabilization during the early stage, which subsides by the late stage; consistently, HIF-1α levels follow this trend. This study elucidates a dynamic and mechanistic picture of metabolic reprogramming in lipopolysaccharide and interferon-γ stimulated macrophages, and provides insights into how changing metabolism can regulate the functional transitions in macrophages over the course of an immune response.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Janeway, C. A. & Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 20, 197–216 (2002).
Mosser, D. M. & Edwards, J. P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 (2008).
Liew, F. Y., Xu, D., Brint, E. K. & O’Neill, L. A. J. Negative regulation of toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 5, 446–458 (2005).
Ivashkiv, L. B. Inflammatory signaling in macrophages: transitions from acute to tolerant and alternative activation states. Eur. J. Immunol. 41, 2477–2481 (2011).
Foster, S. L., Hargreaves, D. C. & Medzhitov, R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 447, 972–978 (2007).
Medvedev, A. E., Kopydlowski, K. M. & Vogel, S. N. Inhibition of lipopolysaccharide-induced signal transduction in endotoxin-tolerized mouse macrophages: dysregulation of cytokine, chemokine, and toll-like receptor 2 and 4 gene expression. J. Immunol. 164, 5564–5574 (2000).
Ziegler-Heitbrock, H. Molecular mechanism in tolerance to lipopolysaccharide. J. Inflamm. 45, 13–26 (1994).
Kumar, V. Targeting macrophage immunometabolism: dawn in the darkness of sepsis. Int. Immunopharmacol. 58, 173–185 (2018).
Navegantes, K. C. et al. Immune modulation of some autoimmune diseases: the critical role of macrophages and neutrophils in the innate and adaptive immunity. J. Transl. Med. 15, 36 (2017).
Jha, A. K. et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 42, 419–430 (2015).
Kelly, B. & O’Neill, L. A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 25, 771–784 (2015).
Lampropoulou, V. et al. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab. 24, 158–166 (2016).
Cordes, T. et al. Immunoresponsive gene 1 and itaconate inhibit succinate dehydrogenase to modulate intracellular succinate levels. J. Biol. Chem. 291, 14274–14284 (2016).
Mills, E. L. et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 167, 457–470.e13 (2016).
Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496, 238–242 (2013).
Liu, P. S. et al. α-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat. Immunol. 18, 985–994 (2017).
Williams, N. C. & O’Neill, L. A. J. A role for the krebs cycle intermediate citrate in metabolic reprogramming in innate immunity and inflammation. Front. Immunol. 9, 141 (2018).
Dominguez-Andres, J. et al. The itaconate pathway is a central regulatory node linking innate immune tolerance and trained immunity. Cell Metab. 29, 211–220.e5 (2018).
Mills, E. L. et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 556, 113–117 (2018).
Bambouskova, M. et al. Electrophilic properties of itaconate and derivatives regulate the IκBζ-ATF3 inflammatory axis. Nature 556, 501–504 (2018).
Medzhitov, R. & Horng, T. Transcriptional control of the inflammatory response. Nat. Rev. Immunol. 9, 692–703 (2009).
Ivashkiv, L. B. Epigenetic regulation of macrophage polarization and function. Trends Immunol. 34, 216–223 (2013).
Novakovic, B. et al. β-Glucan reverses the epigenetic state of LPS-Induced immunological tolerance. Cell 167, 1354–1368.e14 (2016).
Mentch, S. J. et al. Histone methylation dynamics and gene regulation occur through the sensing of one-carbon metabolism. Cell Metab. 22, 861–873 (2015).
Etchegaray, J.-P. & Mostoslavsky, R. Interplay between metabolism and epigenetics: a nuclear adaptation to environmental changes. Mol. Cell 62, 695–711 (2016).
Newsholme, P., Curi, R., Gordon, S. & Newsholme, E. A. Metabolism of glucose, glutamine, long-chain fatty acids and ketone bodies by murine macrophages. Biochem. J. 239, 121–125 (1986).
Fan, J., Krautkramer, K. A., Feldman, J. L. & Denu, J. M. Metabolic regulation of histone post-translational modifications. ACS Chem. Biol. 10, 95–108 (2015).
Munder, M., Eichmann, K. & Modolell, M. Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+T cells correlates with Th1/Th2 phenotype. J. Immunol. 160, 5347–5354 (1998).
Corraliza, I. M., Soler, G., Eichmann, K. & Modolell, M. Arginase induction by suppressors of nitric oxide synthesis (IL-4, IL-10 and PGE2) in murine bone-marrow-derived macrophages. Biochem. Biophys. Res. Commun. 206, 667–673 (1995).
Hard, G. C. Some biochemical aspects of the immune macrophage. Br. J. Exp. Pathol. 51, 97–105 (1970).
Cekic, C. & Linden, J. Purinergic regulation of the immune system. Nat. Rev. Immunol. 16, 177–192 (2016).
Strelko, C. L. et al. Itaconic acid is a mammalian metabolite induced during macrophage activation. J. Am. Chem. Soc. 133, 16386–16389 (2011).
Michelucci, A. et al. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc. Natl Acad. Sci. USA 110, 7820–7825 (2013).
Ackermann, W. W. & Potter, V. R. Enzyme inhibition in relation to chemotherapy. Exp. Biol. Med. 72, 1–9 (1949).
Xiao, M. et al. Inhibition of α-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 26, 1326–1338 (2012).
Safran, M. et al. Mouse model for noninvasive imaging of HIF prolyl hydroxylase activity: assessment of an oral agent that stimulates erythropoietin production. Proc. Natl Acad. Sci. USA 103, 105–110 (2006).
Ramakrishnan, S. K. et al. Loss of von hippel-lindau protein (VHL) increases systemic cholesterol levels through targeting hypoxia-inducible factor 2 and regulation of bile acid homeostasis. Mol. Cell. Biol. 34, 1208–1220 (2014).
Mentch, S. J. & Locasale, J. W. One-carbon metabolism and epigenetics: understanding the specificity. Ann. N. Y. Acad. Sci. 1363, 91–98 (2016).
Chowdhury, R. et al. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep. 12, 463–469 (2011).
Xu, W. et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell 19, 17–30 (2011).
Figueroa, M. E. et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell 18, 553–567 (2010).
Koivunen, P. et al. Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature 483, 484–488 (2012).
Meiser, J. et al. Pro-inflammatory macrophages sustain pyruvate oxidation through pyruvate dehydrogenase for the synthesis of itaconate and to enable cytokine expression. J. Biol. Chem. 291, 3932–3946 (2016).
Kim, J. W., Tchernyshyov, I., Semenza, G. L. & Dang, C. V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 3, 177–185 (2006).
Lu, C. W., Lin, S. C., Chen, K. F., Lai, Y. Y. & Tsai, S. J. Induction of pyruvate dehydrogenase kinase-3 by hypoxia-inducible factor-1 promotes metabolic switch and drug resistance. J. Biol. Chem. 283, 28106–28114 (2008).
Comerford, S. A. et al. Acetate dependence of tumors. Cell 159, 1591–1602 (2014).
Kamphorst, J. J., Chung, M. K., Fan, J. & Rabinowitz, J. D. Quantitative analysis of acetyl-CoA production in hypoxic cancer cells reveals substantial contribution from acetate. Cancer Metab. 2, 23 (2014).
Mills, E. L. & O’Neill, L. A. Reprogramming mitochondrial metabolism in macrophages as an anti-inflammatory signal. Eur. J. Immunol. 46, 13–21 (2016).
Drapier, J. C. & Hibbs, J. B. Murine cytotoxic activated macrophages inhibit aconitase in tumor cells. Inhibition involves the iron-sulfur prosthetic group and is reversible. J. Clin. Invest 78, 790–797 (1986).
Kennedy, M. C., Antholine, W. E. & Beinert, H. An EPR investigation of the products of the reaction of cytosolic and mitochondrial aconitases with nitric oxide. J. Biol. Chem. 272, 20340–30347 (1997).
Shi, L. & Tu, B. P. Acetyl-CoA and the regulation of metabolism: mechanisms and consequences. Curr. Opin. Cell Biol. 33, 125–131 (2015).
Zhang, Z. et al. Identification of lysine succinylation as a new post-translational modification. Nat. Chem. Biol. 7, 58–63 (2011).
Cameron, A. M., Lawless, S. J. & Pearce, E. J. Metabolism and acetylation in innate immune cell function and fate. Semin. Immunol. 28, 408–416 (2016).
Zhou, R., Yazdi, A. S., Menu, P. & Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–226 (2011).
Schieber, M. & Chandel, N. S. ROS function in redox signaling and oxidative stress. Curr. Biol. 24, R453–R462 (2014).
Tong, W. H. et al. TLR-activated repression of Fe-S cluster biogenesis drives a metabolic shift and alters histone and tubulin acetylation. Blood Adv. 2, 1146–1156 (2018).
Mathias, R. A. et al. Sirtuin 4 is a lipoamidase regulating pyruvate dehydrogenase complex activity. Cell 159, 1615–1625 (2014).
Humphries, K. M. & Szweda, L. I. Selective inactivation of α-ketoglutarate dehydrogenase and pyruvate dehydrogenase: reaction of lipoic acid with 4-hydroxy-2-nonenal. Biochemistry 37, 15835–15841 (1998).
O’Brien, M., Chalker, J., Slade, L., Gardiner, D. & Mailloux, R. J. Protein S-glutathionylation alters superoxide/hydrogen peroxide emission from pyruvate dehydrogenase complex. Free Radic. Biol. Med. 106, 302–314 (2017).
McLain, A. L., Cormier, P. J., Kinter, M. & Szweda, L. I. Glutathionylation of α-ketoglutarate dehydrogenase: the chemical nature and relative susceptibility of the cofactor lipoic acid to modification. Free Radic. Biol. Med. 61, 161–169 (2013).
Clasquin, M. F., Melamud, E. & Rabinowitz, J. D. LC-MS data processing with MAVEN: a metabolomic analysis and visualization engine. Curr. Protoc. Bioinformatics 37, 14.11 (2012).
Melamud, E., Vastag, L. & Rabinowitz, J. D. Metabolomic analysis and visualization engine for LC−MS data. Anal. Chem. 82, 9818–9826 (2010).
Allen, E. L. et al. Differential aspartate usage identifies a subset of cancer cells particularly dependent on OGDH. Cell Rep. 17, 876–890 (2016).
Hewitson, K. S., Schofield, C. J. & Ratcliffe, P. J. Hypoxia-Inducible factor prolyl-hydroxylase: purification and assays of PHD2. Methods Enzymol. 435, 25–42 (2007).
Fan, J., Baeza, J. & Denu, J. M. Investigating histone acetylation stoichiometry and turnover rate. Methods Enzymol. 574, 125–148 (2016).
Acknowledgements
This work is primarily supported by the Morgridge Institute for Research (start-up fund for J.F.). Additionally, R.S.E. is supported by NIH grant no. R01DK66600, D.J.P. is supported by NIH grant no. R35GM130294. The authors would like to thank the University of Wisconsin Carbone Cancer Center. This is supported in part by NIH/NCI grant no. P30CA014520—UW Cancer Center Support Grant. The authors would also like to thank Yatrik Shah at the University of Michigan for sharing the ODD-luc mice.
Author information
Authors and Affiliations
Contributions
J.F. and G.L.S. designed the study and analyzed data. E.C.B. performed and analyzed data from DCA treatment experiments. S.V.J. carried out and analyzed data from palmitic-acid labeling studies. F.J.Y. performed some cytokine assays and qPCR. A.R.J. carried out the isolation and analysis of histones. G.L.S. performed all remaining experiments. R.S.E. contributed to the investigation of the role of metabolites in regulating HIF-1α. D.J.P. contributed to the identification of changing lipoylation as a mechanism for PDHC and OGDC regulation. G.L.S. and J.F. wrote the manuscript. R.S.E. and D.J.P. edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information: Primary Handling Editor: Ana Mateus
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–12
Rights and permissions
About this article
Cite this article
Seim, G.L., Britt, E.C., John, S.V. et al. Two-stage metabolic remodelling in macrophages in response to lipopolysaccharide and interferon-γ stimulation. Nat Metab 1, 731–742 (2019). https://doi.org/10.1038/s42255-019-0083-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-019-0083-2
This article is cited by
-
Nitrosylation rewires metabolism
Nature Chemical Biology (2023)
-
Macrophages in immunoregulation and therapeutics
Signal Transduction and Targeted Therapy (2023)
-
Nitric oxide-driven modifications of lipoic arm inhibit α-ketoacid dehydrogenases
Nature Chemical Biology (2023)
-
Pyruvate dehydrogenase operates as an intramolecular nitroxyl generator during macrophage metabolic reprogramming
Nature Communications (2023)
-
Mitochondrial electron transport chain is necessary for NLRP3 inflammasome activation
Nature Immunology (2022)