Abstract
A supramolecular approach is effective to construct topologically complicated molecules with the aid of reversible bond formation. Although topologically complicated molecules have been synthesized for the past three decades, their formation mechanisms have rarely been discussed. Here we report the formation process of a tetranuclear interlocked palladium cage composed of two binuclear cages, which are quadruply interlocked with each other. In the main pathway, the binuclear cages are produced with binuclear partial cages. The ditopic ligand that does not bridge the two palladium(II) ions in the binuclear partial cage then threads into the binuclear cage to afford a tetranuclear partially interlocked cage, with partial conversion of the binuclear cage into the binuclear partial cage. The tetranuclear partially interlocked cage interlocks intramolecularly through repetitive cleavage and formation of Pd(II)–N coordination bonds mediated by a free pyridyl group, finally leading to the tetranuclear interlocked cage.
Similar content being viewed by others
Introduction
Interlocked molecules are nanoscale miniature artworks composed of intertwined molecules that are not connected by covalent bonds but cannot apart from each other without breaking chemical bonds1,2,3,4,5,6,7. Nowadays, such mechanically connected molecules have been recognized as a class of structural motifs for molecular machines beyond simple synthetic targets8,9,10,11,12,13,14. Molecular self-assembly is the best way to efficiently synthesize interlocked molecules. Molecular building blocks spontaneously assemble into a well-organized interlocked structure in high yield as long as the interlocked structure is thermodynamically most stable. The advantage of molecular self-assembly in the formation of interlocked structures is that if the building blocks are incorrectly connected, the reversible chemical bonds employed in molecular self-assembly enable the building blocks to rearrange so that improper species are converted into the final product. A variety of structurally complicated interlocked molecules have been reported by molecular self-assembly for the past three decades15,16,17,18,19,20,21,22,23,24,25. However, how molecular building blocks assemble into such complicated interlocked structures has rarely been discussed26 mainly because the observation of transiently produced intermediates, whose stability is lower than the final product, is quite difficult. Recent progress in the investigation of coordination self-assembly processes by a method that can provide information about intermediates by quantifying all substrates and products instead of intermediates (QASAP: quantitative analysis of self-assembly process) has gradually unveiled ordering processes of supramolecular self-assemblies at the molecular level27,28,29,30.
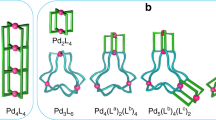
Here we report the self-assembly process of a Pd4L8 interlocked cage (IC)31,32,33,34,35,36,37,38,39, where two molecules of Pd2L4 cages are quadruply interlocked. This is an attempt to understand the formation mechanism of an interlocked coordination assembly. In the self-assembly of the Pd4L8 IC, the Pd2L4 cage is firstly produced with the concomitant formation of a Pd2L4X partial cage (PC) (X: leaving ligand). A ditopic ligand (L) with a free coordination site in PC threads into the Pd2L4 cage or another PC to afford a Pd4L8X partially interlocked cage (PIC). Subsequent intramolecular interlocking in PIC promoted by the coordination of a free ligand (free coordination sites in PC, PIC or Py*) finally gives the thermodynamically most stable Pd4L8 IC.
Results
Monitoring the self-assembly by 1H NMR
The self-assembly process of the Pd4L8 IC from ditopic ligand 1 (Fig. 1)39, which possesses two 3-picolyl groups connected to a benzophenone by ether linkages, and PdPy*4(BF4)2 (Py*: 3-chloropyridine) in CD3NO2 at 298 K was monitored by 1H NMR spectroscopy (Fig. 2 and Supplementary Fig. 1). Right after the self-assembly started, many 1H NMR signals appeared (5 min), which is due to the formation of primitive intermediates. These signals soon disappeared and the signals for the Pd214 cage (signals coloured in red) were observed. The 1H NMR signals of the Pd418 IC (signals coloured in blue) appeared at 5 h with concomitant decrease in the intensity of the signals for the Pd214 cage. The self-assembly finally reached the convergent sample containing 69% of the Pd418 IC and 31% of kinetically trapped species (calculated based on the ditopic ligand 1 by using an internal standard), which could not be converted into the IC even by heating.
Self-assembly process of the interlocked cage. The self-assembly of the Pd418 interlocked cage (IC) takes place through reaction steps (1)–(6): (1) formation of the Pd214Py* partial cage (PC) from PdPy*4(BF4)2 and the ditopic ligand 1 by ligand exchanges between Py* (3-chloropyridine) and the pyridyl groups of 1. As the coordination ability of Py* is slightly weaker than that of the pyridyl groups in 1, the reaction takes place towards the formation of Pd(II)–1 coordination bonds. (2) Intramolecular ligand exchange(s) in the Pd214Py* PC to lead to the Pd214 cage. (3) Conversion of the Pd214 cage to the Pd214Py* PC. (4) Threading of a partially free ditopic ligand in the Pd214Py* PC into the Pd214 cage to lead to a Pd418Py* partially interlocked cage (PIC). (5) Threading of a partially free ditopic ligand in the Pd214Py* PC into another Pd214Py* PC followed by the intramolecular ligand exchange to lead to a Pd418Py* PIC. (6) One of the possible pathways of interlocking in Pd418Py* PIC through repetitive cleavage and formation of Pd(II)–1 coordination bonds to form the Pd418 IC
1H NMR spectra of the self-assembly of the interlocked cage. 1H NMR spectra (500 MHz, CD3NO2, 298 K) of PdPy*4(BF4)2, the ditopic ligand 1, Py* and the reaction mixture for the self-assembly of the Pd418 interlocked cage (IC) from PdPy*4(BF4)2 ([Pd]0 = 1.0 mM) and the ditopic ligand 1 ([1]0 = 2.0 mM) in CD3NO2 at 298 K. The signals coloured in blue, red, green, magenta and brown indicate the Pd418 IC, the Pd214 cage, Py*, the ditopic ligand 1 and PdPy*4(BF4)2, respectively
A very similar result was obtained when the self-assembly was carried out with Pd(CH3CN)4(BF4)2 as a metal source instead of PdPy*4(BF4)2 (Supplementary Figs. 2 and 3); the only difference between the two self-assemblies is that <6% of free ditopic ligand 1 remained until the end in the case of the self-assembly from 1 and Pd(CH3CN)4(BF4)2. In the previous report39, the Pd418 IC was synthesized from 1 and Pd(CH3CN)4(BF4)2 in polar coordinative solvent, DMSO, at a higher temperature of 333 K. Thus, it was found that the Pd418 IC can be assembled in less coordinative solvent at 298 K whether CH3CN or Py* is used as the leaving ligand.
PdPy*4(BF4)2 was used as the Pd(II) ion source so that we could quantify both metal ion source (PdPy*4) and the leaving ligand (Py*) by 1H NMR measurement. During the self-assembly of the Pd418 IC, 1H NMR signals for the Pd214 cage, which is a transient intermediate, were observed, while any other intermediates except for the small signals in the beginning of the self-assembly were not detected by 1H NMR spectroscopy. Thus, the ditopic ligand 1, the Pd418 IC and the Pd214 cage were quantified, from which the existence ratio of the species that cannot be observed by 1H NMR (Int) was determined (gray lines in Fig. 3a, b). In the beginning of the self-assembly, Int was produced in about 80% yield (based on the ditopic ligand 1) and gradually decreased with time. Therefore, the characterization of Int is necessary to understand the self-assembly process of the Pd418 IC. However, none of the signals of Int were observed by 1H NMR spectroscopy, which is the reason for the difficulty in the investigation of self-assembly processes. To solve this problem, in QASAP the average composition of Int (Pd〈a〉1〈b〉Py*〈c〉, 〈a〉, 〈b〉 and 〈c〉 indicate composition ratios) was determined by the existence ratios of all the substrates, products and the Pd214 cage. The time variation of Pd〈a〉1〈b〉Py*〈c〉 enables us to discuss the self-assembly process of the Pd418 IC.
Kinetic data for the self-assembly of the interlocked cage. a The existence ratios of the substrates (1 and PdPy*4(BF4)2), the products (the Pd418 IC and Py*) and the Pd214 cage in the beginning of the self-assembly of the Pd418 IC from 1 and PdPy*4(BF4)2. b The existence ratios of the substrates (1 and PdPy*4(BF4)2), the products (Pd418 IC and Py*) and the Pd214 cage from the beginning till the end of the self-assembly of the Pd418 IC from 1 and PdPy*4(BF4)2. c The change in the (〈n〉, 〈k〉) value with time for the self-assembly of the Pd418 IC from 1 and PdPy*4(BF4)2. Blue crosshairs indicate the (n, k) value of the species, Pda1bPy*c, which is indicated as (a, b, c). d The change in the 〈n〉 value with time for the self-assembly of the Pd418 IC from 1 and PdPy*4(BF4)2. Error bars indicate the standard errors of the means of the existence ratios (a and b) and the (〈n〉, 〈k〉) values (c and d). e The formation of the Pd418 IC from the Pd214 cage in the presence of Py* or 1. f The existence ratios of the Pd418 IC, Py* and the free ditopic ligand 1 for the formation of the Pd418 IC from the Pd214 cage (70% based on 1 initially used) in the presence of Py* or 1. 11.4 equiv. of Py* against the Pd214 cage was added, while 0.57 equiv. of 1 against the Pd214 cage was added. The existence ratios of Py* and 1 are indicated based on Py* and 1 added at t = 0. The inset is a magnified plot of the initial change of the existence ratio of the Pd418 IC
Quantitative analysis of the self-assembly process
The changes in the existence ratios of the substrates (1 and PdPy*4), the products (the Pd418 IC and Py*), the Pd214 cage and all the intermediates except for the Pd214 cage (Int) with time are shown in Fig. 3a, b. The yield of the Pd214 cage reached the maximum (52%) at 5 h and then decreased. The Pd418 IC started to produce at 5 h. Interestingly, after the decrease in the yield of the Pd214 cage, the existence ratio of Int turned to increase for some time and decreased again after 1 day. Slight decrease in the existence ratio of Py* (1.6%) observed during the period when Int increased (5 h to 1 day) indicates that the reverse reaction from the Pd214 cage and Py* to Pd214Py* took place (Pd214 + Py* → Pd214Py*). As the amount of Py* consumed is very small (only 1.6%), the formation of Pd214Py* would be most probable. All the Pd214 cages at 5 h (52%) were finally converted into the Pd418 IC in 69% yield, indicating that at least 75% of the Pd418 IC was derived from the Pd214 cage.
In order to investigate the intermediates for the Pd214 cage and for the Pd418 IC, n–k analysis27,28,29,30 was carried out. In QASAP, two parameters (〈n〉 and 〈k〉) indicating the nature of Int (whose average composition is indicated as Pd〈a〉1〈b〉Py*〈c〉) are defined and used for the characterization of the intermediates. The 〈n〉 value, which is defined as 〈n〉 = (4〈a〉–〈c〉)/〈b〉, indicates the average number of Pd(II) ions binding to a single ditopic ligand, while the 〈k〉 value (〈k〉 = 〈a〉/〈b〉) indicates the ratio between the Pd(II) ions and the ditopic ligands in Int. The change in the (〈n〉, 〈k〉) value with time is shown in Fig. 3c. From 5 to 30 min, the 〈n〉 value increased with time, while the 〈k〉 value decreased, which reflects the incorporation of free ditopic ligands 1 into Int (Fig. 3a). The (〈n〉, 〈k〉) value finally reached (1.85, 0.50) and stayed around there until almost the end of the self-assembly. The Pd214 cage was predominantly produced during the first 5 h, while the Pd418 IC was produced after 5 h. With this in mind, the result that the (〈n〉, 〈k〉) value observed at 15 min (1.76 ± 0.09, 0.52 ± 0.01), is very near to the (n, k) value of a Pd214Py* partial cage (PC), (1.75, 0.5), suggests that the Pd214Py* PC is the long-lived intermediate of the Pd214 cage. The 〈n〉 value at 35 min (1.84) is much larger than the n value of the Pd214Py* PC (1.75) but smaller than that of Pd418Py* (1.875). This suggests that the intermediates for the Pd214 cage (mainly the Pd214Py* PC) and for the Pd418 IC (mainly the Pd418Py* PIC) have already been produced much earlier than when the Pd418 IC begins to produce (5 h). The (〈n〉, 〈k〉) value reached (1.88. 0.5) at 3 days, which indicates that the Pd418Py* PIC, whose (n, k) value is (1.875, 0.5), is the main intermediate of the Pd418 IC.
The self-assembly of the Pd418 IC under the same condition was monitored by ESI-TOF mass spectrometry (Supplementary Fig. 4). The species mainly observed are listed in Table 1. The signals for the Pd214Py* PC could not be found. This is due to the removal of the Py* molecule, whose coordination ability is weaker than 1, during the ionization. Similar results have often been obtained in other Pd(II)-linked coordination self-assemblies40,41,42. The result that the signals for Pd418 were observed instead of that for Pd418Py* can be interpreted in the same way. Interestingly, the signals for Pd418 (m/z = 526.40 for [Pd418(BF4)]7+ and 771.60 for [Pd418(BF4)3]5+) appeared at 30 min, which is much faster than when the 1H NMR signals for the Pd418 IC started to appear (5 h). This result indicates that these mass signals should arise from the Pd418Py* PIC by the removal of the Py* molecule during the ionization. Thus, the Pd418Py* PIC have already been produced by 30 min as the intermediates of the Pd418 IC, which is well consistent with the result that the (〈n〉, 〈k〉) value at 35 min, (1.84, 0.5), is between those of the Pd214Py* PC (1.75, 0.5) and the Pd418Py* PIC (1.875, 0.5) (Fig. 3c).
Transformation mechanism of the Pd214 cage to the Pd418 IC
QASAP for the self-assembly of the Pd418 IC from 1 and PdPy*4(BF4)2 revealed that all the Pd214 cages (52% yield at 5 h determined based on 1) were transformed into the Pd418 IC in 69% yield (based on 1) after the convergence (Fig. 3b). This indicates that the Pd418 IC is mainly produced through the Pd214 cage. Thus, the conversion mechanism from the Pd214 cage to the Pd418 IC was investigated. The Pd214 cage can be isolated from a reaction mixture of 1 ([1]0 = 77.5 mM) and Pd(CH3CN)4(BF4)2 in CH3CN at 343 K as a precipitate39, but once the Pd214 cage is precipitated, the cage is not soluble in any solvent except DMSO39. After several attempts, we found that the reaction of 1 and Pd(CH3CN)4(BF4)2 in CD3NO2 in a 4:2.2 ratio at 343 K, where slightly excess amount of Pd(CH3CN)4(BF4)2 was added, resulted in the selective formation of the Pd214 cage in 70% yield (based on 1) without the formation of the Pd418 IC (Fig. 3e and Supplementary Fig. 5). Although the isolation of the Pd214 cage from this reaction mixture failed, the conversion of the species that remained after the convergence of the self-assembly into the Pd418 IC is negligible because the Pd418 IC was not produced by standing this solution mainly containing the Pd214 cage at 343 K for 4 days. As breaking of Pd(II) coordination bonds does not take place without coordination of a certain ligand to the Pd(II) center to form five-coordinate intermediates and the transition structure through associative mechanism43, 44, the transformation of the Pd214 cage to the Pd418 IC should be promoted by Py* or free ditopic ligand 1.
Upon the addition of Py* (11.4 equiv. against the Pd214 cage, which is equal to 2.0 equiv. against the initial amount of 1) to the above CD3NO2 solution containing the Pd214 cage (70% yield), 2.0 equiv. of Py* molecules against the Pd214 cage were consumed in 1 day (blue solid circles in Fig. 3f). (The 1H NMR spectra and the existence ratios are shown in Supplementary Figs 6 and 7, respectively.) The production of the Pd418 IC started at 3 h after the addition of Py* (blue open circles in inset of Fig. 3f). The Pd418 IC was formed in 64% yield (based on 1) at 12 days, so most of the Pd214 cages (91%) were converted into the Pd418 IC. It is worth noting that after the convergence of the conversion, 1.5 equiv. of Py* were still consumed, suggesting that a part of Py* molecules should be incorporated into the uncharacterized species. This is reasonable because a part of the Pd(II) centers in the uncharacterized species should be occupied with CH3CN molecules, whose coordination ability is weaker than that of Py*. Therefore, only the difference between the maximum consumption (2.0 equiv.) and the consumption after the convergence (1.5 equiv.), 0.5 equiv. of Py*, is the amount of Py* molecules used in the transformation. This suggests that the Pd214Py* PC and the Pd418Py* PIC were produced as the dominant intermediates, which is consistent with the 〈n〉 value of about 1.85 after 20 min in the self-assembly of the Pd418 IC from 1 and PdPy*4 (Fig. 3c). During the time-lag between the addition of Py* and the formation of IC (2 h), formation of the Pd214Py* PC, threading the PC into the Pd214 cage to lead to the Pd418Py* PIC and intramolecular interlocking in the PIC should take place (Fig. 1). The rate of formation of the Pd418 IC from the Pd214 cage in the presence of Py* is slower than that from 1 and PdPy*4 (Fig. 3f). This is because the Pd214Py* PC has already been produced as a part of Int in the self-assembly from 1 and PdPy*4.
The conversion of the Pd214 cage into the Pd418 IC also took place by the addition of 0.57 equiv. of free ditopic ligand 1 against the Pd214 cage (red solid and open circles in Fig. 3f). (The 1H NMR spectra and the existence ratios are shown in Supplementary Figs. 8 and 9, respectively.) As the yield of the Pd418 IC is 70% after the convergence (red open circles in Fig. 3f), all the Pd214 cages were efficiently converted into the Pd418 IC. As observed in the conversion of the Pd214 cage in the presence of Py*, 0.23 equiv. of the added ditopic ligand 1 were consumed after the convergence (red solid circles in Fig. 3f), indicating that a part of ditopic ligands 1 were incorporated into the uncharacterized species. Compared with the addition of Py* instead of 1, the time-lag between the addition of 1 and the formation of IC (1 h) is shorter and the formation of the Pd418 IC took place faster even though the amount of 1 added is much fewer than the amount of Py* added (inset in Fig. 3f). This is probably because the coordination ability of 1 is stronger than that of Py*. The mass signal of (2,5,0) observed during the self-assembly of the Pd418 IC from 1 and PdPy*4 (Table 1) suggests that a part of the Pd418 IC molecules is produced mediated by the Pd215 PC. As mentioned above, 6% of free ditopic ligand 1 remained until the end of the self-assembly of the Pd418 IC from Pd(CH3CN)4(BF4)2 and 1, while the Pd418 IC could not be obtained at all when slightly excess amount of Pd(CH3CN)4(BF4)2 was used. These results indicate that free ditopic ligand 1 plays a role as the catalyst for the conversion of the Pd214 cage into the Pd418 IC in the self-assembly of IC from Pd(CH3CN)4(BF4)2 and 1. As a consequence, species with sufficiently strong coordination ability (free ditopic ligand 1, Py* and the free pyridyl groups in the Pd214Py* PC and the Pd418Py* PIC) can promote the formation of the Pd418Py* PIC and the intramolecular treading in the PIC.
Effect of BF4 – anions on the self-assembly process
As seen in every Pd4L8 IC reported so far, the trapping of three counter anions in between the Pd(II) ions in the Pd4L8 ICs is essential for their formation as templates (Fig. 1)31,32,33,34,35,36,37,38,39, so the effect of the counter anions on the self-assembly process of the Pd418 IC from 1 and PdPy*4(BF4)2 was investigated by 19F NMR spectroscopy (Fig. 4). The signals for the two kinds of BF4– anions trapped in the Pd418 IC were observed at –144.0 ppm (inner) and –142.6 ppm (outer), respectively (Fig. 4a), whose assignment is the same as was reported previously39. These 19F NMR signals for the encapsulated BF4– anions appeared at almost the same time when the 1H NMR signals of the Pd418 IC appeared. Only one signal of BF4– was observed at –152.3 ppm before the formation of the Pd418 IC. This signal is near to the signal of free BF4– (–153.0 ppm) but slightly shifted to downfield. This signal was gradually shifted to upfield with time and finally only the signals for the free and encapsulated BF4– anions in the Pd418 IC were observed. The diffusion coefficient of the BF4– signal at –152.3 ppm determined by 19F DOSY spectroscopy (Supplementary Fig. 10), 1.01 × 10–9 m2 s–1, is larger than that bound in the Pd418 IC (–142.6 and –144.0 ppm), 2.39 × 10–10 m2 s–1 (Supplementary Fig. 11), and smaller than that of free BF4–, 1.36 × 10–9 m2 s–1 (Supplementary Fig. 12). These results indicate that there exist BF4– anions chemically different from the free BF4– and the encapsulated ones in the Pd418 IC, which should be the BF4– anions that interact with the Pd214 cage and/or the intermediates of the Pd418 IC, and that the exchange between these and the free BF4– anions is faster than the NMR time scale at 298 K. In order to characterize these BF4– anions, 19F NMR measurements were carried out at 253 K (9 K higher than the melting point of CD3NO2). Upon decreasing the temperature of the reaction mixture at 6 h to 253 K, the 19F NMR signal at –152.3 ppm observed at 298 K was not divided, indicating that the exchange between the chemically different BF4– anions is still faster than the NMR time scale. Similar 19F NMR spectra showing only one signal at –152.3 and –152.3 ppm at 298 and 253 K, respectively, were observed (Fig 4b, c). The interaction between BF4– anions and the inner space of the Pd214 cage was previously confirmed by 1H NMR titration experiment39. Considering the fact that the Pd214 cage and the intermediates (mainly Pd214Py* PC and Pd418Py* PIC) coexist in the reaction mixture at 6 h, the BF4– anions should interact not only with the Pd214 cage but also with the intermediates. As a consequence, BF4– anions participate in the self-assembly process of the Pd418 IC but are not bound to the intermediates as strongly as to the Pd418 IC.
19F NMR spectra of the self-assembly of the interlocked cage. 19F NMR spectra (470 MHz, CD3NO2) of the reaction mixture for the self-assembly of the Pd418 interlocked cage (IC) from PdPy*4(BF4)2 ([Pd]0 = 1.0 mM) and the ditopic ligand 1 ([1]0 = 2.0 mM) in CD3NO2 at 298 K. a Time variation of the 19F NMR spectra of the reaction mixture measured at 298 K. Signals coloured in blue indicate two kinds of chemically different (inner and outer) BF4– anions trapped in the Pd418 IC. The 19F NMR spectrum of the Pd214 cage is shown as a comparison. b A magnified view of the 19F NMR spectra observed in upfield region for the reaction mixture at 6 h and 7 days with the 19F NMR spectrum of the Pd214 cage measured at 298 K. c A magnified view of the 19F NMR spectra observed in upfield region for the reaction mixture at 6 h and 7 days with the 19F NMR spectrum of the Pd214 cage measured at 253 K
Discussion
In conclusion, the self-assembly process of the Pd418 IC was investigated by QASAP. Although the production of the Pd418 IC was observed by time-dependent 1H NMR spectroscopy and ESI-TOF mass spectrometry, the signals for the intermediates except the Pd214 cage could not be observed due to their lower symmetry and lower stability than the Pd214 and Pd418 cages. Nevertheless, valuable information to discuss the self-assembly process was obtained by QASAP. The self-assembly of the Pd418 IC takes place in three steps. Initially, the Pd214Py* partial cages (PC) were produced, about half of which were converted into the Pd214 cage. Then, threading the Pd214Py* PC into the Pd214 cage leads to the Pd418Py* PICs. Finally, the Pd418 IC is produced by the intramolecular interlocking through repetitive cleavage and formation of Pd(II)–N coordination bonds in the PIC with the aid of free pyridyl ligands (Py* or free pyridyl group in the Pd214Py* PC and the Pd418Py* PIC). Although the Pd418Py* PIC would be produced from two molecules of Pd214Py* PCs, the result that at least 75% of the Pd418 IC is derived from the Pd214 cage indicates that this route is a minor pathway. Considering the fact that the Pd418 IC is a topologically complicated structure where two Pd214 cages are quadruply interlocked, it is expected that many undesired species in which several Pd(II)–pyridyl coordination bonds are mistakenly formed are produced under mild condition (at 298 K), where kinetically controlled products tend to be formed. Contrary to this intuition, the Pd418 IC was assembled in a straightforward way in high yield mainly through the Pd214 cage and the Pd214Py* PC as primary intermediates. ESI-TOF mass spectrometry and n–k analysis suggest that the formation of the Pd418Py* PIC started quickly (at 30 min). On the other hand, 1H NMR measurements indicate that the Pd418 IC appeared after 5 h. During this time-lag, the intramolecular interlocking in the Pd418Py* PIC should take place. Similarly, it takes 3 h to start to produce the Pd418 IC from the Pd214 cage in the presence of Py*. Thus, the intramolecular interlocking in the Pd418Py* PIC is the rate-determining steps of the self-assembly of the Pd418 IC. The Pd418 IC binds three BF4– anions in between the four Pd(II) ions, which stabilize the Pd418 IC. 19F NMR spectroscopy of the reaction mixture indicates that BF4– anions interact with the Pd214 cage and the Pd418Py* PIC, yet the binding of the BF4– anions in these species is as weak as the exchange of the BF4– anions with free BF4– is faster than the NMR time scale. Therefore, the effect of the counter anion on the self-assembly process of the IC is to shift the equilibrium towards the IC by the selective stabilization of the IC.
Methods
General information
1H and 19F NMR spectra were recorded using a Bruker AV-500 (500 MHz) spectrometer. All 1H NMR spectra were referenced using a residual solvent peak, CD3NO2 (δ 4.33), and all 19F NMR spectra were referenced using a peak of n-Bu4NBF4 (δ –153.0) in CD3NO2 at 298 K. Electrospray ionization time-of-flight (ESI-TOF) mass spectra were obtained using a Waters Xevo G2-S Tof mass spectrometer.
Materials
Unless otherwise noted, all solvents and reagents were obtained from commercial suppliers (TCI Co., Ltd., WAKO Pure Chemical Industries Ltd., KANTO Chemical Co., Inc. and Sigma-Aldrich Co.) and were used as received. CD3NO2 was purchased from Acros Organics and used after dehydration with molecular sieves 4 Å. Ditopic ligand 131 and PdPy*4(BF4)245 were prepared according to the literature.
Monitoring the self-assembly of the Pd4 1 8(BF4)8 IC by 1H and 19F NMR
A 2.4 mM solution of [2.2]paracyclophane in CHCl3 (125 μL), which was used as an internal standard, was added to two NMR tubes (tubes I and II) and the solvent was removed in vacuo. A solution of PdPy*4(BF4)2 (12 mM) in CD3NO2 was prepared (solution A). Solution A (50 μL) and CD3NO2 (450 μL) were added to tube I. The exact concentration of PdPy*4(BF4)2 in solution A was determined through the comparison of the signal intensity with [2.2]paracyclophane by 1H NMR. A solution of ditopic ligand, 1, (12 mM) in CHCl3 (100 μL) was added to tube II and the solvent was removed in vacuo. Then CD3NO2 (500 μL) for 1 was added to tube II and the exact amount of 1 in tube II was determined through the comparison of the signal intensity with [2.2]paracyclophane by 1H NMR. 0.50 equiv. (against the amount of ligand 1 in tube II) of solution A (ca. 50 μL; the exact amount was determined based on the exact concentration of solution A and of ligand in tube II) were added to tube II at 263 K. The self-assembly of the Pd418(BF4)8 IC was monitored at 298 K by 1H and 19F NMR spectroscopy. Examples of the 1H NMR spectra are shown in Fig. 2 and Supplementary Fig. 1 and those of the 19F NMR spectra are shown in Fig. 4a, b. The exact ratio of 1 and PdPy*4(BF4)2 was unambiguously determined by the comparison of the integral value of each 1H signal of [2.2]paracyclophane. The amounts of 1, PdPy*4(BF4)2, the Pd214(BF4)4 cage, the Pd418(BF4)8 IC and Py* were quantified by the integral value of each 1H signal against the signal of the internal standard ([2.2]paracyclophane). In order to confirm the reproducibility, the same experiment was carried out three times (runs 1–3). These data, the average values of the existence ratios and the (〈n〉, 〈k〉) values are listed in Supplementary Tables 1–5.
Determination of the existence ratio of each species
The relative integral value of each 1H NMR signal against the internal standard [2.2]paracyclophane is used as the integral value in this description. We define the integral values of the signal for the substrates (1 and PdPy*4), the products (the Pd418 IC and Py*) and the Pd214 cage at each time t as follows; IL(t): 1/2 of the integral value of the a proton in free ligand 1; IM(t): the integral value of the i proton of Py* in [PdPy*4]2+; Icage(t): 1/2 of the integral value of the a proton in the Pd214 cage; IIC(t): the integral value of the ai proton in the Pd418 IC; IPy*(t): the integral value of the i proton of free Py*. (The assignment of signals for the Pd418 IC is shown in Fig. 2 and Supplementary Fig. 1.)
IM(0) was determined based on the exact concentration of solution A determined by 1H NMR and the exact volume of solution A added into tube II.
IL(0) was determined by 1H NMR measurement before the addition of solution A into tube II.
Existence ratio of [PdPy*4]2+: As the total amount of [PdPy*4]2+ corresponds to IM(0), the existence ratio of [PdPy*4]2+ at t is expressed by IM(t)/IM(0).
Existence ratio of 1: As the total amount of free ligand 1 corresponds to IL(0), the existence ratio of 1 at t is expressed by IL(t)/IL(0).
Existence ratio of Py*: As the total amount of Py* corresponds to IM(0), the existence ratio of Py* at t is expressed by IPy*(t)/IM(0).
Existence ratio of the Pd214 cage: As the Pd214 cage is quantified based on 1, the existence ratio of the Pd214 cage at t is expressed by Icage(t)/IL(0).
Existence ratio of the Pd418 IC: As the Pd418 IC is quantified based on 1, the existence ratio of the Pd418 IC at t is expressed by IIC(t)/IL(0).
Existence ratio of the total intermediates not observed by 1H NMR (Int): The existence ratio of the total intermediates not observed by 1H NMR (Int) is determined based on the amount of ligand 1 in the intermediates. Thus, the existence ratio of Int is calculated by subtracting the other species containing 1 (free 1, the cage and IC) from the total amount of 1 (IL(0)). The existence ratio of Int at t is expressed by (IL(0)–IL(t)–Icage(t)–IIC(t))/IL(0).
〈a〉: The total amount of Pd(II) ions corresponds to IM(0)/4. The amount of Pd(II) ions in [PdPy*4]2+ at t corresponds to IM(t)/4. The amount of Pd(II) ions in the cage and IC at t corresponds to Icage(t)/2 and IIC(t)/2, respectively. The amount of Pd(II) ions in Int at t is thus expressed by IM(0)/4–IM(t)/4–Icage(t)/2–IIC(t)/2.
〈b〉: The total amount of ligand 1 corresponds to IL(0). The amount of free ligand 1 at t corresponds to IL(t). The amounts of ligand 1 in the cage and IC at t corresponds to Icage(t) and IIC(t), respectively. The amount of ligand 1 in Int at t is thus expressed by IL(0)–IL(t)–Icage(t) – IIC(t).
〈c〉: The total amount of Py* corresponds to IM(0). The amount of free Py* at t corresponds to IPy*(t). The amount of Py* in [PdPy*4]2+ at t corresponds to IM(t). The amount of Py* in Int at t is thus expressed by IM(0)–IPy*(t)–IM(t).
The 〈n〉 and 〈k〉 values are determined with these 〈a〉, 〈b〉 and 〈c〉 values as follows:
Quantitative analysis of the self-assembly process
In QASAP, all the substrates (1 and PdPy*4(BF4)2), the products (the Pd418(BF4)8 IC and Py*) and the Pd214(BF4)4 cage were quantified by 1H NMR spectroscopy during the self-assembly of the cage and then the amount of the intermediates not observed by 1H NMR (Int) and the average composition of the unobservable intermediates, Pd〈a〉1〈b〉Py*〈c〉, were obtained. The existence ratios of the substrates, the products, the Pd214(BF4)4 cage and Int and 〈a〉, 〈b〉, 〈c〉, 〈n〉 and 〈k〉 with time are listed in Supplementary Tables 1–5 and plotted in Fig. 3.
Self-assembly of the Pd4 1 8(BF4)8 IC from Pd(CH3CN)4(BF4)2 and 1
The self-assembly of the Pd418(BF4)8 IC from Pd(CH3CN)4(BF4)2 ([Pd]0 = 1.0 mM) and 1 ([1]0 = 2.0 mM) was monitored by 1H NMR spectroscopy. The experiment was conducted in the same way as was described in the section “Monitoring the self-assembly of the Pd418(BF4)8 IC by 1H and 19F NMR” with the difference that Pd(CH3CN)4(BF4)2 was used instead of PdPy*4(BF4)2. Because of the difficulty in the quantification of Pd(CH3CN)4(BF4)2 and CH3CN by 1H NMR, only the consumption of 1 and the formation of the Pd214 cage and the Pd418 IC were monitored by 1H NMR at 298 K. 1H NMR spectra are provided in Supplementary Fig. 2 and the existence ratios of the substrates (1 and PdPy*4(BF4)2), the products (the Pd418 IC and Py*), the Pd214 cage and Int are plotted in Supplementary Fig. 3.
Mass spectrometry
A solution of PdPy*4(BF4)2 ([Pd]0 = 0.95 mM) and 1 ([1]0 = 1.9 mM) were mixed in CD3NO2 (550 μL). At each time point, 25 μL of the reaction mixture was taken, diluted with CH3NO2 (500 μL), filtered through a membrane filter (pore size: 0.20 μm) and injected in the mass spectrometer (measurement condition: capillary/1.5 kV; sampling cone/30 V; source offset/80 V; source/80 °C; sesolvation/150 °C; cone gas/50 L h–1; desolvation gas/800 L h–1) with 5.0 μL/min flow rate to obtain ESI-TOF mass spectra (Supplementary Fig. 4). A list of predominantly observed species is shown in Table 1.
Preparation of the Pd2 1 4(BF4)4 cage
The exact amount of 1 in an NMR tube was determined through the comparison of the signal intensity with [2.2]paracyclophane by 1H NMR. Then the self-assembly of the Pd214(BF4)4 cage was carried out from Pd(CH3CN)4(BF4)2 ([Pd]0 = 1.1 mM) and 1 ([1]0 = 2.0 mM) in CD3NO2 (550 μL) at 343 K in the same way as was described in the section “Monitoring the self-assembly of the Pd418(BF4)8 IC by 1H and 19F NMR”. After 12 h, the Pd214(BF4)4 cage was obtained in 70% yield (determined by 1H NMR based on the internal standard ([2.2]paracyclophane)). 1H NMR spectra are provided in Supplementary Fig. 5.
Formation of the Pd4 1 8 IC from the Pd2 1 4 cage in the presence of Py*
The Pd214(BF4)4 cage was prepared in the same way as described in the section “Preparation of the Pd214(BF4)4 cage” in 70% yield (determined by 1H NMR based on the internal standard ([2.2]paracyclophane)). Then 2.0 equiv. of Py* (2.0 μmol) against the initial amount of 1 (1.0 μmol) was added into the reaction mixture and then the reaction of the Pd214 cage and Py* was monitored by 1H NMR at 298 K. 1H NMR spectra are provided in Supplementary Fig. 6 and the existence ratios of the substrates (the Pd214 cage and Py*), the products (the Pd418 IC) and Int are plotted in Supplementary Fig. 7.
Formation of the Pd4 1 8 IC from the Pd2 1 4 cage in the presence of 1
The Pd214(BF4)4 cage was prepared in the same way as described in the section “Preparation of the Pd214(BF4)4 cage” in 70% yield (determined by 1H NMR based on the internal standard ([2.2]paracyclophane)). Then 0.1 equiv. of 1 (0.10 μmol) against the initial amount of 1 (1.0 μmol) was added into the reaction mixture and then the reaction of the Pd214 cage and 1 was monitored by 1H NMR at 298 K. 1H NMR spectra are provided in Supplementary Fig. 8 and the existence ratios of the substrates (the Pd214 cage and 1), the products (the Pd418 IC) and Int are plotted in Supplementary Fig. 9.
19F DOSY NMR measurements
19F DOSY NMR spectra of the reaction mixture for the self-assembly from PdPy*4(BF4)2 ([Pd]0 = 1.0 mM) and 1 ([1]0 = 2.0 mM) in CD3NO2 recorded at 6 h and at 7 days and of a solution of n-Bu4NBF4 in CD3NO2 are shown in Supplementary Figs. 10–12, respectively. The diffusion coefficients and the hydrodynamic radii of the observed species are summarized in Supplementary Table 6. The hydrodynamic radii, r, were determined by the Stokes–Einstein equation (3), where D is a diffusion coefficient obtained from 19F DOSY NMR spectroscopy, kB is Boltzmann constant, T is the measurement temperature, which was applied to be 298 K, and η is the viscosity of solvent, which was applied to be 0.61 mPa s (CH3NO2).
Data availability
The authors declare that all the other data supporting the findings of this study are available within the article and its supplementary information files and from the corresponding author upon request.
References
Bruns, C. J. & Stoddart, J. F. The Nature of the Mechanical Bond: from Molecules to Machines (John Wiley & Sons, Inc., New York, 2016).
Amabilino, D. B. & Stoddart, J. F. Interlocked and intertwined structures and superstructures. Chem. Rev. 95, 2725–2828 (1995).
Du, S. M., Stollar, B. D. & Seeman, N. C. A synthetic DNA molecule in three knotted topologies. J. Am. Chem. Soc. 117, 1194–1200 (1995).
Mao, C., Sun, W. & Seeman, N. C. Assembly of borromean rings from DNA. Nature 386, 137–138 (1997).
Raymo, F. M. & Stoddart, J. F. Interlocked macromolecules. Chem. Rev. 99, 1643–1664 (1999).
Amabilino, D. B. & Pérez-García, L. Topology in molecules inspired, seen and represented. Chem. Soc. Rev. 38, 1562–1571 (2009).
Liu, Y., O’Keeffe, M., Treacy, M. M. J. & Yaghi, O. M. The geometry of periodic knots, polycatenanes and weaving from a chemical perspective: a library for reticular chemistry. Chem. Soc. Rev. 47, 4642–4664 (2018).
Pease, A. R. et al. Switching devices based on interlocked molecules. Acc. Chem. Res. 34, 433–444 (2001).
Leigh, D. A., Wong, J. K. Y., Dehez, F. & Zerbetto, F. Unidirectional rotation in a mechanically interlocked molecular rotor. Nature 424, 174–179 (2003).
Balzani, V., Credi, A., Silvi, S. & Venturi, M. Artificial nanomachines based on interlocked molecular species: recent advances. Chem. Soc. Rev. 35, 1135–11149 (2006).
Champin, B., Mobian, P. & Sauvage, J.-P. Transition metal complexes as molecular machine prototypes. Chem. Soc. Rev. 36, 358–366 (2007).
van Dongen, S. F. M., Cantekin, S., Elemans, J. A. A. W., Rowan, A. E. & Nolte, R. J. M. Functional interlocked systems. Chem. Soc. Rev. 43, 99–122 (2014).
Jester, S.-S. & Famulok, M. Mechanically interlocked DNA nanostructures for functional devices. Acc. Chem. Res. 47, 1700–1709 (2014).
Chang, J.-C. et al. Mechanically interlocked daisy-chain-like structures as multidimensional molecular muscles. Nat. Chem. 9, 128–134 (2017).
Dietrich-Buchecker, C. O. & Sauvage, J.-P. A synthetic molecular trefoil knot. Angew. Chem. Int. Ed. Engl. 28, 189–192 (1989).
Hasenknopf, B., Lehn, J.-M., Kneisel, B. O., Baum, G. & Fenske, D. Self-assembly of a circular double helicate. Angew. Chem. Int. Ed. Engl. 35, 1838–1840 (1996).
Fujita, M. Self-assembly of [2]catenanes containing metals in their backbones. Acc. Chem. Res. 32, 53–61 (1999).
Chichak, K. S. et al. Molecular borromean rings. Science 304, 1308–1312 (2004).
Guo, J., Mayers, P. C., Breault, G. A. & Hunter, C. A. Synthesis of a molecular trefoil knot by folding and closing on an octahedral coordination template. Nat. Chem. 2, 218–222 (2010).
Forgan, R. S., Sauvage, J.-P. & Stoddart, J. F. Chemical topology: complex molecular knots, links, and entanglements. Chem. Rev. 111, 5434–5464 (2011).
Leong, W. L. & Vittal, J. J. One-dimensional coordination polymers: complexity and diversity in structures, properties, and applications. Chem. Rev. 111, 688–764 (2011).
Chambron, J.-C. & Sauvage, J.-P. Topologically complex molecules obtained by transition metal templation: it is the presentation that determines the synthesis strategy. New J. Chem. 37, 49–57 (2013).
Lewis, J. E. M., Beer, P. D., Loeb, S. J. & Goldup, S. M. Metal ions in the synthesis of interlocked molecules and materials. Chem. Soc. Rev. 46, 2577–2591 (2017).
Sawada, T., Yamagami, M., Ohara, K., Yamaguchi, K. & Fujita, M. Peptide [4]catenane by folding and assembly. Angew. Chem. Int. Ed. 55, 4519–4522 (2016).
Zhang, L. et al. Stereoselective synthesis of a composite knot with nine crossings. Nat. Chem. 10, 1083–1088 (2018).
Ponnuswamy, N., Cougnon, F. B. L., Clough, J. M., Pantos, G. D. & Sanders, J. K. M. Discovery of an organic trefoil knot. Science 338, 783–785 (2012).
Tsujimoto, Y., Kojima, T. & Hiraoka, S. Rate-determining step in the self-assembly process of supramolecular coordination capsules. Chem. Sci. 5, 4167–4172 (2014).
Hiraoka, S. What do we learn from the molecular self-assembly process? Chem. Rec. 15, 1144–1147 (2015).
Hiraoka, S. Unresolved issues that remain in molecular self-assembly. Bull. Chem. Soc. Jpn. 91, 957–978 (2018).
Hiraoka, S. Self-assembly processes of Pd(II)- and Pt(II)-linked discrete self-assemblies revealed by QASAP. Isr. J. Chem. https://doi.org/10.1002/ijch.201800073 In the press (2018).
Fukuda, M., Sekiya, R. & Kuroda, R. A quadruply stranded metallohelicate and its spontaneous dimerization into an interlocked metallohelicate. Angew. Chem. Int. Ed. 47, 706–710 (2008).
Freye, S. et al. Allosteric binding of halide anions by a new dimeric interpenetrated coordination cage. Angew. Chem. Int. Ed. 51, 2191–2194 (2012).
Freye, S. et al. Template control over dimerization and guest selectivity of interpenetrated coordination cages. J. Am. Chem. Soc. 135, 8476–8479 (2013).
Frank, M. et al. Assembly and stepwise oxidation of interpenetrated coordination cages based on phenothiazine. Angew. Chem. Int. Ed. 52, 10102–10106 (2013).
Löffler, S. et al. Triggered exchange of anionic for neutral guests inside a cationic coordination cage. J. Am. Chem. Soc. 137, 1060–1063 (2015).
Zhu, R., Lübben, J., Dittrich, B. & Clever, G. H. Stepwise halide-triggered double and triple catenation of self-assembled coordination cages. Angew. Chem. Int. Ed. 54, 2796–2800 (2015).
Sekiya, R., Fukuda, M. & Kuroda, R. Site-selective anion recognition of an interlocked dimer. Org. Biomol. Chem. 15, 4328–4335 (2017).
Clever, G. H. & Punt, P. Cation–anion arrangement patterns in self-assembled Pd2L4 and Pd4L8 coordination cages. Acc. Chem. Res. 50, 2233–2243 (2017).
Sekiya, R., Fukuda, M. & Kuroda, R. Anion-directed formation and degradation of an interlocked metallohelicate. J. Am. Chem. Soc. 134, 10987–10997 (2012).
Kai, S. et al. The effect of solvent and coordination environment of metal source on the self-assembly pathway of a Pd(II)-mediated coordination capsule. Inorg. Chem. 56, 12652–12663 (2017).
Kai, S. et al. Self-assembly process of a Pd2L4 capsule: steric interactions between neighboring components favor the formation of large intermediates. Chem. Eur. J. 24, 3965–3969 (2018).
Tateishi, T., Kojima, T. & Hiraoka, S. Chiral self-sorting process in the self-assembly of homochiral coordination cages from axially chiral ligands. Commun. Chem. 1, 20 (2018).
Tobe, M. L. & Burgess, J. Inorganic Reaction Mechanisms (Longman: London, U.K., 1999).
Cooper, J. & Ziegler, T. A density functional study of SN2 substitution at square-planar platinum complexes. Inorg. Chem. 41, 6614–6622 (2002).
Tateishi, T. et al. Self-assembly of a Pd4L8 double-walled square partly takes place through the formation of kinetically trapped species. Eur. J. Inorg. Chem. 1192–1197 (2018).
Acknowledgements
We thank Dr. Ryo Sekiya for helpful discussion about the preparation of the Pd214 cage. This research was supported by JSPS Grants-in-Aid for Scientific Research on Innovative Areas “Dynamical Ordering of Biomolecular Systems for Creation of Integrated Functions” (25102001 and 25102005) and The Asahi Glass Foundation.
Author information
Authors and Affiliations
Contributions
T.T., Y.Y., T.K., S.T. and S.H. conceived the project. S.H. prepared the manuscript and all the authors discussed the results and commented on the manuscript. T.T. and Y.Y. synthesized the ditopic ligands 1. T.T. carried out all the NMR and ESI-TOF mass measurements. T.T., T.K. and S.H. analyzed the experimental data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tateishi, T., Yasutake, Y., Kojima, T. et al. Self-assembly process of a quadruply interlocked palladium cage. Commun Chem 2, 25 (2019). https://doi.org/10.1038/s42004-019-0123-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-019-0123-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.