Abstract
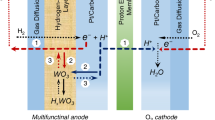
Repetitive start-up and shut-down events in polymer electrolyte membrane fuel cells for automotive applications lead to serious corrosion of the cathode due to an instantaneous potential jump that results from unintended air leakage into the anodic flow field followed by a parasitic oxygen reduction reaction (ORR) on the anode. Here we report a solution to the cathode corrosion issue during the start-up/shut-down events whereby intelligent catalyst design is used to selectively promote the hydrogen oxidation reaction (HOR) while concomitantly suppressing the ORR on the anode. Platinum thin layers supported on hydrogen tungsten bronze (Pt/HxWO3) suppressed the ORR by converting themselves into an insulator following exposure to oxygen, while selectively promoting the HOR by regaining metallic conductivity following subsequent exposure to hydrogen. The HOR-selective electrocatalysis imparted by a metal–insulator transition in Pt/HxWO3 demonstrated a remarkably enhanced durability of membrane electrode assemblies compared to those with commercial Pt/C catalysts.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the plots in this paper and other findings of this study are available from the corresponding author on reasonable request.
Change history
17 February 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41929-020-00501-0
References
Yu, P. T. et al. The impact of carbon stability on PEM fuel cell startup and shutdown voltage degradation. ECS Trans. 3, 797–809 (2006).
Borup, R. et al. Scientific aspects of polymer electrolyte fuel cell durability and degradation. Chem. Rev. 107, 3904–3951 (2007).
de Bruijn, F. A., Dam, V. A. T. & Janssen, G. J. M. Review: durability and degradation issues of PEM fuel cell components. Fuel Cells 8, 3–22 (2008).
Kaspar, R. B. et al. Reverse-current decay in hydroxide exchange membrane fuel cells. J. Electrochem. Soc. 163, F377–F383 (2016).
Reiser, C. A. et al. A reverse-current decay mechanism for fuel cells. Electrochem. Solid-State Lett. 8, A273–A276 (2005).
Tang, H. et al. PEM fuel cell cathode carbon corrosion due to the formation of air/fuel boundary at the anode. J. Power Sources 158, 1306–1312 (2006).
Baumgartner, W. R. et al. Polarization study of a PEMFC with four reference electrodes at hydrogen starvation conditions. J. Power Sources 182, 413–421 (2008).
Patterson, T. W. & Darling, R. M. Damage to the cathode catalyst of a PEM fuel cell caused by localized fuel starvation. Electrochem. Solid-State Lett. 9, A183–A185 (2006).
Yousfi-Steiner, N., Moçotéguy, P. H., Candusso, D. & Hissel, D. A review on polymer electrolyte membrane fuel cell catalyst degradation and starvation issues: causes, consequences and diagnostic for mitigation. J. Power Sources 194, 130–145 (2009).
Ishigami, Y. et al. Corrosion of carbon supports at cathode during hydrogen/air replacement at anode studied by visualization of oxygen partial pressures in a PEFC—start-up/shut-down simulation. J. Power Sources 196, 3003–3008 (2011).
Linse, N. et al. Quantitative analysis of carbon corrosion during fuel cell start-up and shut-down by anode purging. J. Power Sources 219, 240–248 (2012).
Chan, S. H. et al. Gas purging effect on the degradation characteristic of a proton exchange membrane fuel cell with dead-ended mode operation II. Under different operation pressures. Energy 131, 50–57 (2017).
Yu, Y. et al. Effect of gas shutoff sequences on the degradation of proton exchange membrane fuel cells with dummy load during startup and shutdown cycles. Electrochim. Acta 71, 181–193 (2012).
Chen, Y.-S. et al. A purge strategy for proton exchange membrane fuel cells under varying-load operations. Int. J. Hydrog. Energy 41, 12369–12376 (2016).
Selvaganesh, S. V. et al. Graphitic carbon as durable cathode-catalyst support for PEFCs. Fuel Cells 11, 372–384 (2011).
Wang et al. Durability investigation of carbon nanotube as catalyst support for proton exchange membrane fuel cell. J. Power Sources 158, 154–159 (2006).
Watanabe, M. et al. Characterization of Pt catalysts on Nb-doped and Sb-doped SnO2–δ support materials with aggregated structure by rotating disk electrode and fuel cell measurements. Electrochim. Acta 110, 316–324 (2013).
Uchida, M. et al. Novel strategy to mitigate cathode catalyst degradation during air/air startup cycling via the atmospheric resistive switching mechanism of a hydrogen anode with a platinum catalyst supported on tantalum-doped titanium dioxide. J. Power Sources 294, 292–298 (2015).
Hara, M. et al. Electrochemical and Raman spectroscopic evaluation of Pt/graphitized carbon black catalyst durability for the start/stop operating condition of polymer electrolyte fuel cells. Electrochim. Acta 70, 171–181 (2012).
Uchida, M. et al. Durability of Pt catalysts supported on graphitized carbon-black during gas-exchange start-up operation similar to that used for fuel cell vehicles. J. Electrochem. Soc. 163, F644–F650 (2016).
Dubau, L. et al. A review of PEM fuel cell durability: materials degradation, local heterogeneities of aging and possible mitigation strategies. WIREs Energy Eviron. 3, 540–560 (2014).
Srivastava, R. & Strasser, P. Oxygen evolution co-catalysts at fuel cell cathodes for degradation mitigation during simulated start-up shut-down cycles. ECS Trans. 25, 565–571 (2009).
Jang, S.-E. & Kim, H. Effect of water electrolysis catalysts on carbon corrosion in polymer electrolyte membrane fuel cells. J. Am. Chem. Soc. 132, 14700–14701 (2010).
Genorio, B. et al. Selective catalysts for the hydrogen oxidation and oxygen reduction reactions by patterning of platinum with calix[4]arene molecules. Nat. Mater. 9, 998–1003 (2010).
Genorio, B. et al. Tailoring the selectivity and stability of chemically modified platinum nanocatalysts to design highly durable anodes for PEM fuel cells. Angew. Chem. Int. Ed. Engl. 50, 5468–5472 (2011).
Kim, Y.-T. et al. Hydrogen oxidation-selective electrocatalysis by fine tuning of Pt ensemble sites to enhance the durability of automotive fuel cells. ChemSusChem 10, 489–493 (2017).
Ogi, T. et al. Electrospun Pt/SnO2 nanofibers as an excellent electrocatalysts for hydrogen oxidation reaction with ORR-blocking characteristic. Catal. Commun. 33, 11–14 (2013).
Romano-Rodriguez, A. et al. Insight into the role of oxygen diffusion in the sensing mechanisms of SnO2 nanowires. Adv. Funct. Mater. 18, 2990–2994 (2008).
Granqvist, C. G. Electrochromic tungsten oxide films: review of progress 1993–1998. Sol. Energy Mater. Sol. Cells 60, 201–262 (2000).
Georg, A., Graf, W., Neumann, R. & Wittwer, V. The role of water in gasochromic WO films. Thin Solid Films 384, 269–275 (2001).
Tseung, A. C. C. & Chen, K. Y. Hydrogen spill-over effect on Pt/WO3 anode catalysts. Catal. Today 38, 439–443 (2014).
Xi, Y. et al. Mechanism of hydrogen spillover on WO3(001) and formation of HxWO3 (x = 0.125, 0.25, 0.375, and 0.5). J. Phys. Chem. C. 118, 494–501 (2014).
Shi, J. et al. Electrochemical catalytic activity for the hydrogen oxidation of mesoporous WO3 and WO3/C composites. J. Mater. Chem. 18, 3575–3580 (2008).
Kawazoe, H. et al. Formation of hydrogen tungsten bronze by proton implantation. Mater. Res. Bull. 34, 115–121 (1999).
Kamal, H. et al. Influence of proton insertion on the conductivity, structural and optical properties of amorphous and crystalline electrochromic WO3 films. Phys. B: Condens. Matter 349, 192–205 (2004).
Adzic, R. R. et al. Palladium monolayer and palladium alloy electrocatalysts for oxygen reduction. Langmuir 22, 10409–10415 (2006).
Tseung, A. C. C. et al. High performance, platinum activated tungsten oxide fuel cell electrodes. Nature 222, 556–558 (1969).
Fabregat-Santiago, F. et al. Dynamic processes in the coloration of WO3 by lithium insertion. J. Electrochem. Soc. 148, E302–E309 (2001).
Bisquert, J. & Vikhrenko, V. S. Analysis of the kinetics of ion intercalation. Two state model describing the coupling of solid state ion diffusion and ion binding processes. Electrochim. Acta 47, 3977–3988 (2002).
Kim, D.-J. et al. A study on the hydrogen intercalation into rf-magnetron sputtered amorphous WO3 film using cyclic voltammetry combined with electrochemical quartz crystal microbalance technique. Solid State Ion. 109, 81–87 (1998).
Lee, S.-H. et al. Crystalline WO3 nanoparticles for highly improved electrochromic applications. Adv. Mater. 18, 763–766 (2006).
Wen, R.-T. et al. Eliminating degradation and uncovering ion-trapping dynamics in electrochromic WO3 thin films. Nat. Mater. 14, 996–1001 (2015).
Wen, R.-T. et al. Sustainable rejuvenation of electrochromic WO3 films. ACS Appl. Mater. Interfaces 7, 28100–28104 (2015).
Mortimer, R. J. Electrochromic material. Annu. Rev. Mater. Res. 41, 241–268 (2011).
Reich, S. et al. A possible 2D HxWO3 Superconductor with a Tc of 120 K. J. Supercond. Nov. Magn. 22, 343–346 (2009).
Tong, Q. et al. Rhenium-promoted Pt/WO3/ZrO2: an efficient catalyst for aqueous glycerol hydrogenolysis under reduced H2 pressure. RSC Adv. 6, 86663–86672 (2016).
Li, Haizeng et al. Self-seeded growth of nest-like hydrated tungsten trioxide film directly on FTO substrate for highly enhanced electrochromic performance. J. Mater. Chem. A 2, 11305–11310 (2014).
Hsu, W.-C. et al. Hydrogen sensing characteristics of an electrodeposited WO3 thin film gasochromic sensor activated by Pt catalyst. Thin Solid Films 516, 407–411 (2007).
Nørskov, J. K. et al. Changing the activity of electrocatalysts for oxygen reduction by tuning the surface electronic structure. Angew. Chem. 118, 2963–2967 (2006).
Nørskov, J. K. et al. Modeling the electrochemical hydrogen oxidation and evolution reactions on the basis of density functional theory calculations. J. Phys. Chem. C 114, 18182–18197 (2010).
Bard, A. J. et al. in Electrochemical Methods: Fundamentals and Applications 351 (Wiley, 1980).
Garsany, Y. et al. Experimental methods for quantifying the activity of platinum electrocatalysts for the oxygen reduction reaction. Anal. Chem. 82, 6321–6328 (2010).
Kresse, G. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 169 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Hammer, B., Hansen, L. B. & Nørskov, J. K. Improved adsorption energetics within density-fuctional theory using revised Perdew–Burke–Ernzerhof functionals. Phys. Rev. B 59, 7413–7421 (1999).
Acknowledgements
This work was supported by the National Research Foundation (NRF) of Korea grants (nos. 2019M3D1A1079306, 2019M3E6A1064521). V.S. and N.M.M. were supported by the U.S. Department of Energy, Office of Energy Efficiency and Renewable Energy, Hydrogen and Fuel Cells Technologies Office.
Author information
Authors and Affiliations
Contributions
Y.-T.K. conceived and designed the experiments. S.-M.J., S.-W.Y., J.-H.K., S.-H.Y, J.P., S.L., S.H.C. and S.C.C. performed the experiments. Y.-T.K., S.H.J., J.L., Y.J., J. Son, V.S. and N.M.M. analysed the data. Y.-T.K., J. Snyder and N.M.M. co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs 1–30 and Tables 1–3.
Rights and permissions
About this article
Cite this article
Jung, SM., Yun, SW., Kim, JH. et al. Selective electrocatalysis imparted by metal–insulator transition for durability enhancement of automotive fuel cells. Nat Catal 3, 639–648 (2020). https://doi.org/10.1038/s41929-020-0475-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-020-0475-4
This article is cited by
-
Direct prediction of gas adsorption via spatial atom interaction learning
Nature Communications (2023)
-
Tuning metal-support interaction of Pt-based electrocatalysts for hydrogen energy conversion
Science China Chemistry (2023)
-
PtNi-W/C with Atomically Dispersed Tungsten Sites Toward Boosted ORR in Proton Exchange Membrane Fuel Cell Devices
Nano-Micro Letters (2023)
-
Advance in 3D self-supported amorphous nanomaterials for energy storage and conversion
Nano Research (2023)
-
Highly dispersed Pt species anchored on W18O49 nanowires mediate efficient and durable hydrogen evolution in acidic water
Science China Materials (2022)