Abstract
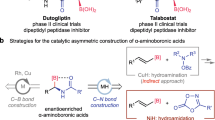
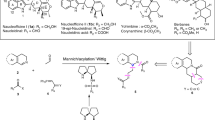
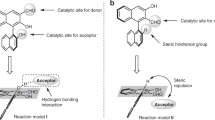
β2- and β3-amino acids are important chiral building blocks for the design of new pharmaceuticals and peptidomimetics. Here, we report a straightforward regio- and enantiodivergent access to these compounds using a one-pot reaction composed of sparteine-mediated enantioselective lithiation of a Boc-1,3-oxazinane, transmetallation to zinc and direct or migratory Negishi coupling with an organic electrophile. The regioselectivity of the Negishi coupling was highly ligand-controlled and switchable to obtain the C4- or the C5-functionalized product exclusively. High enantioselectivities were achieved on a broad range of examples, and a catalytic version in chiral diamine was developed using the (+)-sparteine surrogate. Selected C4- and C5-functionalized Boc-1,3-oxazinanes were subsequently converted to highly enantioenriched β2- and β3-amino acids with the (R) or (S) configuration, depending on the sparteine enantiomer employed in the lithiation step.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data supporting the findings of this study are available in the Supplementary Information or from the corresponding author upon request. The Supplementary Information contains full details on the synthesis and characterization of compounds. CCDC 1913804 (compound (R)-11a) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/structures/.
References
Kudo, F., Miyanaga, A. & Eguchi, T. Biosynthesis of natural products containing β-amino acids. Nat. Prod. Rep. 31, 1056–1073 (2014).
Ma, J. S. Unnatural amino acids in drug discovery. Chim. Oggi 21, 65–68 (2003).
Juaristi, E. and Soloshonok, V. A. Enantioselective Synthesis of β-amino Acids (Wiley-Interscience, 2005).
Steer, D. L., Lew, R. A., Perlmutter, P., Smith, A. I. & Aguilar, M.-I. β-amino acids: versatile peptidomimetics. Curr. Med. Chem. 9, 811–822 (2002).
Cabrele, C., Martinek, T. A., Reiser, O. & Berlicki, L. Peptides containing β-amino acid patterns: challenges and successes in medicinal chemistry. J. Med. Chem. 57, 9718–9739 (2014).
Vasseur, A., Bruffaerts, J. & Marek, I. Remote functionalization through alkene isomerization. Nat. Chem. 8, 209–219 (2016).
Baudoin, O. Selectivity control in the palladium-catalyzed cross-coupling of alkyl nucleophiles. Chima 70, 768–772 (2016).
Sommer, H., Juliá-Hernańdez, F., Martin, R. & Marek, I. Walking metals for remote functionalization. ACS Cent. Sci. 4, 153–165 (2018).
Millet, A., Larini, P., Clot, E. & Baudoin, O. Ligand-controlled β-selective C(sp3)–H arylation of N-Boc-piperidines. Chem. Sci. 4, 2241–2247 (2013).
Werner, E. W., Mei, T.-S., Burckle, A. J. & Sigman, M. S. Enantioselective Heck arylations of acyclic alkenyl alcohols using a redox-relay strategy. Science 338, 1455–1458 (2012).
Correia, C. R. D., Oliveira, C. C., Salles, A. G. Jr. & Santos, E. A. F. The first examples of enantioselective Heck–Matsuda reaction: arylation of unactivated cyclic olefins using chiral bisoxazolines. Tetrahedron Lett. 53, 3325–3328 (2012).
Mei, T.-S., Patel, H. H. & Sigman, M. S. Enantioselective construction of remote quaternary stereocenters. Nature 508, 340–344 (2014).
Li, L., Romano, C. & Mazet, C. Palladium-catalyzed long-range deconjugative isomerization of highly substituted α,β-unsaturated carbonyl compounds. J. Am. Chem. Soc. 138, 10344–10350 (2016).
Beak, P. & Kerrick, S. T. Asymmetric deprotonations: enantioselective syntheses of 2-substituted (tert-butoxycarbonyl)pyrrolidines. J. Am. Chem. Soc. 113, 9708–9710 (1991).
Campos, K. R., Klapars, A., Waldman, J. H. & Dormer, P. G. Chen, C.-Y. Enantioselective, palladium-catalyzed α-arylation of N-Boc-pyrrolidine. J. Am. Chem. Soc. 128, 3538–3539 (2006).
Bailey, W. F., Beak, P., Kerrick, S. T., Ma, S. & Wiberg, K. B. An experimental and computational investigation of the enantioselective deprotonation of Boc-piperidine. J. Am. Chem. Soc. 124, 1889–1896 (2002).
Stead, D. et al. Asymmetric deprotonation of N-Boc piperidine: react IR monitoring and mechanistic aspects. J. Am. Chem. Soc. 132, 7260–7261 (2010).
Beak, P. and Yum, E. K. Lithiation of N-Boc-2-methyltetrahydro-1,3-oxazine: a synthetic equivalent for 1-lithio-3-hydroxy-1-propylamine. J. Org. Chem. 58, 823–824 (1993).
McDermott, B. P., Campbell, A. D. & Ertan, A. First example of s-BuLi/(–)-sparteine-mediated chiral deprotonation of a piperazine and proof of the sense of induction. Synlett 6, 875–879 (2008).
Maulide, N., Peng, B., Mateus Afonso, C. A. & Machado Frade, R. F. Process for converting lupanine into sparteine. Patent WO 2014191261 (2014).
Zhang, K.-F., Christoffel, F. & Baudoin, O. Barbier–Negishi coupling of secondary alkyl bromides with triflates and nonaflates. Angew. Chem. Int. Ed. 57, 1982–1986 (2018).
Dixon, A. J., McGrath, M. J. & O’Brien, P. Synthesis of (+)-(1R,2S,9S)-11-methyl-7,11-diazatricyclo[7.3.1.02,7]tridecane, a (+)-sparteine surrogate. Org. Synth. 83, 141–154 (2006).
Royal, T., Baumgartner, Y. & Baudoin, O. Enantioselective α-arylation of O-carbamates via sparteine-mediated lithiation and Negishi cross-coupling. Org. Lett. 19, 166–169 (2017).
Rottländer, M. & Knochel, P. Palladium-catalyzed cross-coupling reactions with aryl nonaflates: a practical alternative to aryl triflates. J. Org. Chem. 63, 203–208 (1998).
Renaudat, A. et al. The palladium-catalyzed β arylation of carboxylic esters. Angew. Chem. Int. Ed. 49, 7261–7265 (2010).
Millet, A., Dailler, D., Larini, P. & Baudoin, O. Ligand-controlled α- and β-arylation of acyclic N-Boc-amines. Angew. Chem. Int. Ed. 53, 2678–2682 (2014).
Dupuy, S., Zhang, K.-F., Goutierre, A.-S. & Baudoin, O. Terminal-selective functionalization of alkyl chains by regioconvergent cross-coupling. Angew. Chem. Int. Ed. 55, 14793–14797 (2016).
Hoppe, D., Paetow, M. & Hintze, F. Stereodivergent enantioselective synthesis by exploiting unusually large kinetic H/D isotope effects on deprotonation. Angew. Chem. Int. Ed. Engl. 32, 394–396 (1993).
Gallagher, D. J. & Beak, P. Complex-induced proximity effects: evidence for a prelithiation complex and a rate-determining deprotonation in the asymmetric lithiation of Boc-pyrrolidine by an i-PrLi/(–)-sparteine complex. J. Org. Chem. 60, 7092–7093 (1995).
Meisner, J. & Kästner, J. Atom tunneling in chemistry. Angew. Chem. Int. Ed. 55, 5400–5413 (2016).
Old, D. W., Wolfe, J. P. & Buchwald, S. L. A highly active catalyst for palladium-catalyzed cross-coupling reactions: room-temperature Suzuki couplings and amination of unactivated aryl chlorides. J. Am. Chem. Soc. 120, 9722–9723 (1998).
McGrath, M. J. & O’Brien, P. Catalytic asymmetric deprotonation using a ligand exchange approach. J. Am. Chem. Soc. 127, 16378–16379 (2005).
Barker, G. et al. Enantioselective, palladium-catalyzed α-arylation of N-Boc pyrrolidine: in situ React IR spectroscopic monitoring, scope, and synthetic applications. J. Org. Chem. 76, 5936–5953 (2011).
Firth, J. D., Canipa, S. J., Ferris, L. & O’Brien, P. Gram‐scale synthesis of the (–)‐sparteine surrogate and (–)‐sparteine. Angew. Chem. Int. Ed. 57, 223–226 (2018).
Lait, S. M., Rankic, D. A. & Keay, B. A. 1,3-aminoalcohols and their derivatives in asymmetric organic synthesis. Chem. Rev. 107, 767–796 (2007).
Carlsen, P. H. J., Katsuki, T., Martin, V. S. & Sharpless, K. B. A greatly improved procedure for ruthenium tetraoxide catalyzed oxidations of organic compounds. J. Org. Chem. 46, 3936–3938 (1981).
De Mico, A., Margarita, R., Parlanti, L., Vescovi, A. & Piancatelli, G. A versatile and highly selective hypervalent iodine (III)/2,2,6,6-tetramethyl-1-piperidinyloxyl-mediated oxidation of alcohols to carbonyl compounds. J. Org. Chem. 62, 6974–6977 (1997).
Acknowledgements
This work was financially supported by the Swiss National Science Foundation (grant no. 200021_165987) and the University of Basel. We thank A. Prescimone, University of Basel, for X-ray diffraction analysis, D. Häussinger, University of Basel, for NMR experiments, S. Mittelheisser and M. Pfeffer, University of Basel, for mass spectrometry analysis and J. Rotzler and F. Bächle (Solvias AG), for fruitful discussions.
Author information
Authors and Affiliations
Contributions
W.L. and K.-F.Z. designed and performed the experiments, analysed the experimental data and prepared the Supplementary Information. O.B. directed the investigations and prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Table 1, Supplementary Figures 1–2, Supplementary References
Compound (R)-11a
Crystallographic data for compound (R)-11a
Rights and permissions
About this article
Cite this article
Lin, W., Zhang, KF. & Baudoin, O. Regiodivergent enantioselective C–H functionalization of Boc-1,3-oxazinanes for the synthesis of β2- and β3-amino acids. Nat Catal 2, 882–888 (2019). https://doi.org/10.1038/s41929-019-0336-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-019-0336-1
This article is cited by
-
Stereoselective synthesis through remote functionalization
Nature Synthesis (2022)
-
Facile access to chiral non-natural amino acids
Nature Catalysis (2019)