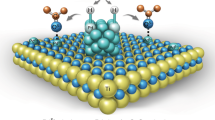
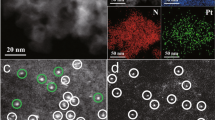
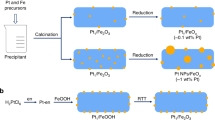
Abstract
The catalytic activities of supported metal nanoparticles can be tuned by appropriate design of synthesis strategies. Each step in a catalyst synthesis method can play an important role in preparing the most efficient catalyst. Here we report the careful manipulation of the post-synthetic heat treatment procedure—together with control over the metal loading—to prepare a highly efficient 0.2 wt% Pt/TiO2 catalyst for the chemoselective hydrogenation of 3-nitrostyrene. For Pt/TiO2 catalysts with 0.2 and 0.5 wt% loading levels, reduction at 450 °C induces the coverage of TiOx over Pt nanoparticles through a strong metal–support interaction, which is detrimental to their catalytic activities. However, this can be avoided by following calcination treatment with reduction (both at 450 °C), allowing us to prepare an exceptionally active catalyst. Detailed characterization has revealed that the peripheral sites at the Pt/TiO2 interface are the most likely active sites for this hydrogenation reaction.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Information on the data supporting the results presented here, including how to access them, can be found in the Cardiff University data catalogue at https://doi.org/10.17035/d.2019.0079744472.
Change history
11 November 2019
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
03 August 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41929-020-00500-1
References
Wang, L. et al. Single-site catalyst promoters accelerate metal-catalyzed nitroarene hydrogenation. Nat. Commun. 9, 1362 (2018).
Therrien, A. J. et al. An atomic-scale view of single-site Pt catalysis for low-temperature CO oxidation. Nat. Catal. 1, 192–198 (2018).
Vile, G., Albani, D., Almora-Barrios, N., Lopez, N. & Perez-Ramirez, J. Advances in the design of nanostructured catalysts for selective hydrogenation. Chem. Cat. Chem. 8, 21–33 (2016).
Blaser, H. U., Steiner, H. & Studer, M. Selective catalytic hydrogenation of functionalized nitroarenes: an update. Chem. Cat. Chem. 1, 210–221 (2009).
Song, J. J. et al. Review on selective hydrogenation of nitroarene by catalytic, photocatalytic and electrocatalytic reactions. Appl. Catal. B 227, 386–408 (2018).
Rylander, P. N. Hydrogenation Methods (Academic Press, London, 1990).
Nishimura, S. Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis (Wiley-VCH, New York, 2001).
Bellamy, F. D. & Ou, K. Selective reduction of aromatic nitro-compounds with stannous chloride in non-acidic and non-aqueous medium. Tetrahedron Lett. 25, 839–842 (1984).
Basu, M. K., Becker, F. F. & Banik, B. K. Ultrasound-promoted highly efficient reduction of aromatic nitro compounds to the aromatic amines by samarium/ammonium chloride. Tetrahedron Lett. 41, 5603–5606 (2000).
Burawoy, A. & Critchley, J. P. Electronic spectra of organic molecules and their interpretation-V: effect of terminal groups containing multiple bonds on the K-bands of conjugated systems. Tetrahedron 5, 340–351 (1959).
Corma, A., Serna, P., Concepcion, P. & Calvino, J. J. Transforming non-selective into chemoselective metal catalysts for the hydrogenation of substituted nitroaromatics. J. Am. Chem. Soc. 130, 8748–8753 (2008).
Arnold, H., Döbert, F. & Gaube, J. In Handbook of Heterogeneous Catalysis (eds G., Ertl, Knözinger, H., Schüth, F. and Weitkamp, J.) (Wiley-VCH, 2008).
Shi, Q. X., Lu, R. W., Jin, K., Zhang, Z. X. & Zhao, D. F. Simple and eco-friendly reduction of nitroarenes to the corresponding aromatic amines using polymer-supported hydrazine hydrate over iron oxide hydroxide catalyst. Green. Chem. 8, 868–870 (2006).
Shi, Q., Lu, R., Lu, L., Fu, X. & Zhao, D. Efficient reduction of nitroarenes over nickel-iron mixed oxide catalyst prepared from a nickel-iron hydrotalcite precursor. Adv. Synth. Catal. 349, 1877–1881 (2007).
Wang, X. K. et al. Heterogeneous catalytic transfer partial-hydrogenation with formic acid as hydrogen source over the Schiff-base modified gold nano-catalyst. Catal. Lett. 147, 517–524 (2017).
Pogorelic, I. et al. Rapid, efficient and selective reduction of aromatic nitro compounds with sodium borohydride and Raney nickel. J. Mol. Catal. A 274, 202–207 (2007).
Rahman, A. & Jonnalagadda, S. B. Swift and selective reduction of nitroaromatics to aromatic amines with Ni–boride–silica catalysts system at low temperature. Catal. Lett. 123, 264–268 (2008).
Corma, A. & Serna, P. Chemoselective hydrogenation of nitro compounds with supported gold catalysts. Science 313, 332–334 (2006).
Wei, H. et al. FeOx-supported platinum single-atom and pseudo-single-atom catalysts for chemoselective hydrogenation of functionalized nitroarenes. Nat. Commun. 5, 5634–5642 (2014).
Wei, H. et al. Remarkable effect of alkalis on the chemoselective hydrogenation of functionalized nitroarenes over high-loading Pt/FeOx catalysts. Chem. Sci. 8, 5126–5131 (2017).
Sankar, M. et al. Synthesis of stable ligand-free gold-palladium nanoparticles using a simple excess anion method. ACS Nano 6, 6600–6613 (2012).
Zanella, R., Louis, C., Giorgio, S. & Touroude, R. Crotonaldehyde hydrogenation by gold supported on TiO2: structure sensitivity and mechanism. J. Catal. 223, 328–339 (2004).
Cargnello, M. et al. Efficient removal of organic ligands from supported nanocrystals by fast thermal annealing enables catalytic studies on well-defined active phases. J. Am. Chem. Soc. 137, 6906–6911 (2015).
Freakley, S. J. et al. Palladium–tin catalysts for the direct synthesis of H2O2 with high selectivity. Science 351, 965–968 (2016).
Chen, C., Cao, J. J., Cargnello, M., Fornasiero, P. & Gorte, R. J. High-temperature calcination improves the catalytic properties of alumina-supported Pd@ceria prepared by self assembly. J. Catal. 306, 109–115 (2013).
Riggs, W. M. X-ray photoelectron spectrometry of platinum compounds. Anal. Chem. 44, 830–832 (1972).
Moulder, J. F., Stickle, W. F., Sobol, P. E. & Bomben, K. D. Handbook of X-ray Photoelectron Spectroscopy (Perkin-Elmer Corporation, 1992).
Matos, J. et al. In situ coarsening study of inverse micelle-prepared Pt nanoparticles supported on gamma-Al2O3: pre-treatment and environmental effects. Phys. Chem. Chem. Phys. 14, 11457–11467 (2012).
Kim, G. J., Kwon, D. W. & Hong, S. C. Effect of Pt particle size and valence state on the performance of Pt/TiO2 catalysts for CO oxidation at room temperature. J. Phys. Chem. C 120, 17996–18004 (2016).
Zimmermann, R. et al. Electronic structure of 3D-transition-metal oxides: on-site Coulomb repulsion versus covalency. J. Phys. Condens. Matter 11, 1657–1682 (1999).
Naumkin, A. V., Kraut-Vass, A., Gaarenstroom, S. W. & Powell, C. J. NIST X-ray Photoelectron Spectroscopy Database 20, Version 4.1 (National Institute of Standards and Technology, 2017); http://srdata.nist.gov/xps/
Janin, E. et al. Hydrogen adsorption on the Pt(111)(3 × 3)R30° Sn surface alloy studied by high resolution core level photoelectron spectroscopy. Appl. Surf. Sci. 99, 371–378 (1996).
Brown, M., Peierls, R. E. & Stern, E. A. White lines in X-ray absorption. Phys. Rev. B 15, 738–744 (1977).
Koningsberger, D. & Prins, R. X-ray Absorption: Principles, Applications, Techniques of EXAFS, SEXAFS, and XANES (Wiley, 1988).
Penner-Hahn, J. E. X-ray absorption spectroscopy in coordination chemistry. Coord. Chem. Rev. 190, 1101–1123 (1999).
de Groot, F. High-resolution X-ray emission and X-ray absorption spectroscopy. Chem. Rev. 101, 1779–1808 (2001).
Yoshida, H., Nonoyama, S., Yazawa, Y. & Hattori, T. Quantitative determination of platinum oxidation state by XANES analysis. Phys. Scr. T115, 813–815 (2005).
Hyde, T. I. et al. X-ray absorption spectroscopic studies of platinum speciation in fresh and road aged light-duty diesel vehicle emission control catalysts. Platin. Met. Rev. 55, 233–245 (2011).
Kim, M. Y., You, Y. S., Han, H. S. & Seo, G. Preparation of highly dispersed platinum catalysts impregnated on titania-incorporated silica support. Catal. Lett. 120, 40–47 (2008).
Beale, A. M. & Weckhuysen, B. M. EXAFS as a tool to interrogate the size and shape of mono and bimetallic catalyst nanoparticles. Phys. Chem. Chem. Phys. 12, 5562–5574 (2010).
Raskó, J. CO-induced surface structural changes of Pt on oxide-supported Pt catalysts studied by DRIFTS. J. Catal. 217, 478–486 (2003).
Zafeiratos, S., Papakonstantinou, G., Jacksic, M. M. & Neophytides, S. G. The effect of Mo oxides and TiO2 support on the chemisorption features of linearly adsorbed CO on Pt crystallites: an infrared and photoelectron spectroscopy study. J. Catal. 232, 127–136 (2005).
DeRita, L. et al. Catalyst architecture for stable single atom dispersion enables site-specific spectroscopic and reactivity measurements of CO adsorbed to Pt atoms, oxidized Pt clusters, and metallic Pt clusters on TiO2. J. Am. Chem. Soc. 139, 14150–14165 (2017).
Thang, H. V., Pacchioni, G., DeRita, L. & Christopher, P. Nature of stable single atom Pt catalysts dispersed on anatase TiO2. J. Catal. 367, 104–114 (2018).
Stakheev, A. Y., Shpiro, E. S., Tkachenko, O. P., Jaeger, N. I. & Schulz-Ekloff, G. Evidence for monatomic platinum species in H-ZSM-5 from FTIR spectroscopy of chemisorbed CO. J. Catal. 169, 382–388 (1997).
Tauster, S. J., Fung, S. C. & Garten, R. L. Strong metal–support interactions. Group 8 noble metals supported on Titanium Dioxide. J. Am. Chem. Soc. 100, 170–175 (1978).
Vannice, M. A. & Sen, B. Metal support effects on the intramolecular selectivity of crotonaldehyde hydrogenation over platinum. J. Catal. 115, 65–78 (1989).
Baker, L. R. et al. Furfuraldehyde hydrogenation on titanium oxide-supported platinum nanoparticles studied by sum frequency generation vibrational spectroscopy: acid–base catalysis explains the molecular origin of strong metal–support interactions. J. Am. Chem. Soc. 134, 14208–14216 (2012).
Boronat, M. & Corma, A. Origin of the different activity and selectivity toward hydrogenation of single metal Au and Pt on TiO2 and bimetallic Au-Pt/TiO2 catalysts. Langmuir 26, 16607–16614 (2010).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
EXCURV98 (CCLRC Daresbury Laboratory, 1998).
Acknowledgements
M.M. and A.J.B. acknowledge MAXNET Energy consortium for funding. A.J.B. also acknowledges the EPRSC Centre for Doctoral Training in Catalysis (grant no. EP/L016443/1). M.S. and Q.H. thank Cardiff University for their respective University Research Fellowships and RQ acknowledges Chinese Scholarship Council (CSC) funding for his stay at Cardiff University. C.J.K. gratefully acknowledges funding from the National Science Foundation Major Research Instrumentation programme (grant no. MRI/DMR-1040229). S.M.A. thanks the Saudi Arabian government for his PhD scholarship. The authors thank the UK Catalysis Hub for allocating beamtime slots through the UK Catalysis Hub BAG allocation for X-ray acquisition of the absorption spectroscopic data at the Diamond synchrotron facility (sp15151). We are also indebted to P. Wells for acquisition of the X-ray absorption spectroscopic data at the Diamond synchrotron facility. We thank Y. Odarchenko for his assistance during CO chemisorption measurements. The authors thank the Diamond Light Source for access to the electron Physical Science Imaging Centre (ePSIC instrument E01 and proposal no. MG22776), which contributed to the results presented here.
Author information
Authors and Affiliations
Contributions
M.M., A.J.B. and R.Q. performed the catalyst preparation and catalyst testing under the supervision of M.S., N.D., S.F. and X.G. S.M.A., Q.H. and C.J.K. carried out electron microscopy studies of the catalyst. D.J.M. performed the XPS characterization of the catalysts. E.G. and A.M.B. analysed the X-ray absorption spcectroscopy data. D.B. suggested the kinetics experiments presented in this article. M.S. conceived this idea and supervised the project. G.J.H. directed this project. All authors contributed to the data analysis and drafting of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Macino, M., Barnes, A.J., Althahban, S.M. et al. Tuning of catalytic sites in Pt/TiO2 catalysts for the chemoselective hydrogenation of 3-nitrostyrene. Nat Catal 2, 873–881 (2019). https://doi.org/10.1038/s41929-019-0334-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-019-0334-3
This article is cited by
-
Photochemical tuning of dynamic defects for high-performance atomically dispersed catalysts
Nature Materials (2024)
-
Interplay between geometric and electronic structures of Pt entities over TiO2 for CO oxidation
Science China Chemistry (2024)
-
Enhancing alkyne semi-hydrogenation through engineering metal-support interactions of Pd on oxides
Nano Research (2024)
-
Selective photoelectrochemical oxidation of glucose to glucaric acid by single atom Pt decorated defective TiO2
Nature Communications (2023)
-
Reverse oxygen spillover triggered by CO adsorption on Sn-doped Pt/TiO2 for low-temperature CO oxidation
Nature Communications (2023)