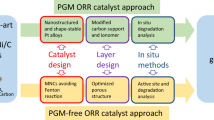
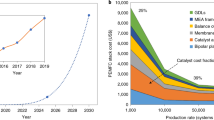
Proton exchange membrane fuel cells can use hydrogen and air to power clean electric vehicles. However, technical barriers including high cost, limited lifetime and insufficient power density limit their broad applications. Advanced cathode catalysts for the kinetically-sluggish oxygen reduction reaction (ORR) in acidic media are essential for overcoming these barriers. Here, we highlight breakthroughs, challenges and future directions for both platinum group metal (PGM) and PGM-free ORR cathode catalysts. Among PGM catalysts, highly-ordered PtM intermetallic nanostructures exhibit enhanced activity and stability relative to PtM random alloys. Carbon supports, with optimal balance between graphitization degree and porosity, play an important role in enhancing overall performance. Among PGM-free catalysts, transition metal and nitrogen co-doped carbons (M-N-C) perform best. However, degradation at practical voltages (>0.6 V) still prevents their practical application. For all catalysts, translating intrinsic activity and stability into device performance requires electrodes with robust three-phase interfaces for effective charge and mass transfer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Thompson, S. T. et al. Electrocat: DOE’s approach to PGM-free catalyst and electrode R&D. Solid State Ionics 319, 68–76 (2018). A brief summary provides a perspective on PGM-free catalysts for transportation and application from the US DOE point of view.
Wang, X. X., Prabhakaran, V., He, Y., Shao, Y. & Wu, G. Iron-free cathode catalysts for proton-exchange-membrane fuel cells: Cobalt catalysts and the peroxide mitigation approach. Adv. Mater. 31, 1805126 (2019).
Adzic, R. R. Platinum monolayer electrocatalysts: tunable activity, stability, and self-healing properties. Electrocatalysis 3, 163–169 (2012).
Chattot, R. et al. Surface distortion as a unifying concept and descriptor in oxygen reduction reaction electrocatalysis. Nat. Mater. 17, 827–833 (2018). Surface distortion as a novel structural descriptor is proposed to rationalize electrocatalytic properties of PtNi/C nanocatalysts.
Gan, L., Cui, C., Rudi, S. & Strasser, P. Core-shell and nanoporous particle architectures and their effect on the activity and stability of Pt ORR electrocatalysts. Top. Catal. 57, 236–244 (2014).
Gómez-Marín, A. M. & Feliu, J. M. Oxygen reduction on nanostructured platinum surfaces in acidic media: Promoting effect of surface steps and ideal response of Pt (111). Catal. Today 244, 172–176 (2015).
Spendelow, J. S. Advanced electro-catalysts through crystallographic enhancement (US DOE, 2017); https://www.hydrogen.energy.gov/pdfs/review18/fc161_spendelow_2018_o.pdf (2018).
Kongkanand, A. & Mathias, M. F. The priority and challenge of high-power performance of low-platinum proton-exchange membrane fuel cells. Chem. Lett. 7, 1127–1137 (2016).A review provides understanding of cathode catalyst layer and ionomer design with low-Pt loading from a fuel cell vehicle application point of view.
Sasaki, K. et al. Core-protected platinum monolayer shell high-stability electrocatalysts for fuel-cell cathodes. Angew. Chem. Int. Ed. 49, 8602–8607 (2010).
Banham, D. & Ye, S. Current status and future development of catalyst materials and catalyst layers for proton exchange membrane fuel cells: an industrial perspective. ACS Energy Lett. 2, 629–638 (2017).
Stamenkovic, V. et al. Changing the activity of electrocatalysts for oxygen reduction by tuning the surface electronic structure. Angew. Chem. Int. Ed. 45, 2897–2901 (2006).
Oezaslan, M., Hasché, F. & Strasser, P. Pt-based core–shell catalyst architectures for oxygen fuel cell electrodes. J. Phys. Chem. Lett. 4, 3273–3291 (2013).
Wu, J. & Yang, H. Platinum-based oxygen reduction electrocatalysts. Acc. Chem. Res. 46, 1848–1857 (2013).
Luo, M. & Guo, S. Strain-controlled electrocatalysis on multimetallic nanomaterials. Nat. Rev. Mater. 2, 17059 (2017).
Stamenkovic, V. R. et al. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 6, 241–247 (2007).
Cho, K. Y. et al. Molybdenum-doped PdPt@Pt core-shell octahedra supported by ionic block copolymer-functionalized graphene as a highly active and durable oxygen reduction electrocatalyst. ACS Appl. Mater. Interfaces 9, 1524–1535 (2017).
Zhang, C., Sandorf, W. & Peng, Z. Octahedral Pt2CuNi uniform alloy nanoparticle catalyst with high activity and promising stability for oxygen reduction reaction. ACS Catal. 5, 2296–2300 (2015).
Huang, X. et al. High-performance transition metal-doped Pt3Ni octahedra for oxygen reduction reaction. Science 348, 1230–1234 (2015).
Yano, H., Watanabe, M., Iiyama, A. & Uchida, H. Particle-size effect of Pt cathode catalysts on durability in fuel cells. Nano Energy 29, 323–333 (2016).
Strasser, P. Catalysts by platonic design. Science 349, 379–380 (2015).
Erikson, H., Sarapuu, A., Tammeveski, K., Solla-Gullón, J. & Feliu, J. M. Shape-dependent electrocatalysis: Oxygen reduction on carbon-supported gold nanoparticles. ChemElectroChem 1, 1338–1347 (2014).
Kuzume, A., Herrero, E. & Feliu, J. M. Oxygen reduction on stepped platinum surfaces in acidic media. J. Electroanal. Chem. 599, 333–343 (2007).
Stamenkovic, V. R. et al. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science 315, 493–497 (2007). An experimental and theoretical study on the catalytic activity of different lattice planes of Pt 3 Ni, suggesting that the Pt 3 Ni(111) surface is more active than Pt(111) because of an unusual d-band centre position.
Marković, N. M., Schmidt, T. J., Stamenković, V. & Ross, P. N. Oxygen reduction reaction on Pt and Pt bimetallic surfaces: A selective review. Fuel Cells 1, 105–116 (2001).
Jiang, K. et al. Efficient oxygen reduction catalysis by subnanometer Pt alloy nanowires. Sci. Adv. 3, e1601705 (2017).
Bu, L. et al. A general method for multimetallic platinum alloy nanowires as highly active and stable oxygen reduction catalysts. Adv. Mater. 27, 7204–7212 (2015).
Luo, M. et al. Stable high-index faceted Pt skin on Zigzag-like PtFe nanowires enhances oxygen reduction catalysis. Adv. Mater. 30, 1705515 (2018).
Li, M. et al. Ultrafine jagged platinum nanowires enable ultrahigh mass activity for the oxygen reduction reaction. Science 354, 1414–1419 (2016).
Sun, X. et al. Core/shell Au/CuPt nanoparticles and their dual electrocatalysis for both reduction and oxidation reactions. J. Am. Chem. Soc. 136, 5745–5749 (2014).
Corona, B., Howard, M., Zhang, L. & Henkelman, G. Computational screening of core@shell nanoparticles for the hydrogen evolution and oxygen reduction reactions. J. Chem. Phys. 145, 244708 (2016).
Ye, W. et al. Enhanced O2 reduction on atomically thin Pt-based nanoshells by integrating surface facet, interfacial electronic, and substrate stabilization effects. Nano Res. 11, 3313–3326 (2018).
Shen, L.-L., Zhang, G.-R., Miao, S., Liu, J. & Xu, B.-Q. Core–shell nanostructured Au@NimPt2 electrocatalysts with enhanced activity and durability for oxygen reduction reaction. ACS Catal. 6, 1680–1690 (2016).
Luo, S., Tang, M., Shen, P. K. & Ye, S. Atomic-scale preparation of octopod nanoframes with high-index facets as highly active and stable catalysts. Adv. Mater. 29, 1601687 (2017).
Wang, X. et al. Pt-based icosahedral nanocages: using a combination of {111} facets, twin defects, and ultrathin walls to greatly enhance their activity toward oxygen reduction. Nano Lett. 16, 1467–1471 (2016).
Park, J., Wang, H., Vara, M. & Xia, Y. Platinum cubic nanoframes with enhanced catalytic activity and durability toward oxygen reduction. ChemSusChem 9, 2855–2861 (2016).
Kwon, H. et al. Dendrite-embedded platinum–nickel multiframes as highly active and durable electrocatalyst toward the oxygen reduction reaction. Nano Lett. 18, 2930–2936 (2018).
Zhang, L. et al. Platinum-based nanocages with subnanometer-thick walls and well-defined, controllable facets. Science 349, 412–416 (2015).
Peng, X., Zhao, S., Omasta, T. J., Roller, J. M. & Mustain, W. E. Activity and durability of Pt–Ni nanocage electocatalysts in proton exchange membrane fuel cells. Appl. Catal. B 203, 927–935 (2017).
Xie, S. et al. Atomic layer-by-layer deposition of Pt on Pd nanocubes for catalysts with enhanced activity and durability toward oxygen reduction. Nano Lett. 14, 3570–3576 (2014).
Antolini, E. Alloy vs. Intermetallic compounds: Effect of the ordering on the electrocatalytic activity for oxygen reduction and the stability of low temperature fuel cell catalysts. Appl. Catal. B 217, 201–213 (2017).
Xiao, W., Lei, W., Gong, M., Xin, H. L. & Wang, D. Recent advances of structurally ordered intermetallic nanoparticles for electrocatalysis. ACS Catal. 8, 3237–3256 (2018).
Jung, W. S. & Popov, B. N. New method to synthesize highly active and durable chemically ordered fct-PtCo cathode catalyst for PEMFCs. ACS Appl. Mater. Interfaces 9, 23679–23686 (2017).
Choi, D. S., Robertson, A. W., Warner, J. H., Kim, S. O. & Kim, H. Low-temperature chemical vapor deposition synthesis of Pt-Co alloyed nanoparticles with enhanced oxygen reduction reaction catalysis. Adv. Mater. 28, 7115–7122 (2016).
Cai, Y., Gao, P., Wang, F. & Zhu, H. Carbon supported chemically ordered nanoparicles with stable Pt shell and their superior catalysis toward the oxygen reduction reaction. Electrochim. Acta 245, 924–933 (2017).
Bu, L. et al. Surface engineering of hierarchical platinum-cobalt nanowires for efficient electrocatalysis. Nat. Commun. 7, 11850 (2016).
Wang, D. et al. Structurally ordered intermetallic platinum–cobalt core-shell nanoparticles with enhanced activity and stability as oxygen reduction electrocatalysts. Nat. Mater. 12, 81–87 (2013). Pt 3 Co@Pt nanoparticles with ordered Pt 3 Co core and Pt shell structure showed enhanced activity and stability for the ORR in acidic media.
Du, X. X., He, Y., Wang, X. X. & Wang, J. N. Fine-grained and fully ordered intermetallic PtFe catalyst with largely enhanced catalytic activity and durability. Energy Environ. Sci. 9, 2623–2632 (2016).
Wang, D. et al. Morphology and activity tuning of Cu3Pt/C ordered intermetallic nanoparticles by selective electrochemical dealloying. Nano Lett. 15, 1343–1348 (2015).
Lang, X.-Y. et al. Mesostructured intermetallic compounds of platinum and non-transition metals for enhanced electrocatalysis of oxygen reduction reaction. Adv. Funct. Mater. 25, 230–237 (2015).
Cui, Z., Chen, H., Zhou, W., Zhao, M. & Disalvo, F. J. Structurally ordered Pt3Cr as oxygen reduction electrocatalyst: ordering control and origin of enhanced stability. Chem. Mater. 27, 7538–7545 (2015).
Bu, L. et al. Biaxially strained PtPb/Pt core/shell nanoplate boosts oxygen reduction catalysis. Science 354, 1410–1414 (2016).
Arumugam, B. et al. Enhanced activity and durability for the electroreduction of oxygen at a chemically ordered intermetallic PtFeCo catalyst. RSC Adv. 4, 27510–27517 (2014).
Zhu, H., Cai, Y., Wang, F., Gao, P. & Cao, J. Scalable preparation of the chemically ordered Pt–Fe–Au nanocatalysts with high catalytic reactivity and stability for oxygen reduction reactions. ACS Appl. Mater. Interfaces 10, 22156–22166 (2018).
Kim, J., Rong, C., Lee, Y., Liu, J. P. & Sun, S. From core/shell structured FePt/Fe3O4/MgO to ferromagnetic FePt nanoparticles. Chem. Mater. 20, 7242–7245 (2008).
Kim, J., Lee, Y. & Sun, S. Structurally ordered FePt nanoparticles and their enhanced catalysis for oxygen reduction reaction. J. Am. Chem. Soc. 132, 4996–4997 (2010).
Li, Q. et al. New approach to fully ordered fct-FePt nanoparticles for much enhanced electrocatalysis in acid. Nano Lett. 15, 2468–2473 (2015).
Chung, D. Y. et al. Highly durable and active PtFe nanocatalyst for electrochemical oxygen reduction reaction. J. Am. Chem. Soc. 137, 15478–15485 (2015).
Bu, L. et al. PtPb/PtNi intermetallic core/atomic layer shell octahedra for efficient oxygen reduction electrocatalysis. J. Am. Chem. Soc. 139, 9576–9582 (2017).
Li, J. et al. Hard-magnet L10-CoPt nanoparticles advance fuel cell catalysis. Joule 3, 124–135 (2018). An ordered intermetallic CoPt catalyst was studied in both acidic aqueous electrolyte and solid-state MEAs with exceptionally enhanced activity and stability, exceeding DOE 2020 targets for PEMFCs’ transportation applications.
Wang, L. et al. Tunable intrinsic strain in two-dimensional transition metal electrocatalysts. Science 363, 870–874 (2019).
Kim, H. Y. et al. Self-supported mesostructured Pt-based bimetallic nanospheres containing an intermetallic phase as ultrastable oxygen reduction electrocatalysts. Small 12, 5347–5353 (2016).
Wang, D. et al. Tuning oxygen reduction reaction activity via controllable dealloying: A model study of ordered Cu3Pt/C intermetallic nanocatalysts. Nano Lett. 12, 5230–5238 (2012).
Gummalla, M. et al. Effect of particle size and operating conditions on Pt3Co PEMFC cathode catalyst durability. Catalysts 5, 926–948 (2015).
Jia, Q. et al. Improved oxygen reduction activity and durability of dealloyed PtCox catalysts for proton exchange membrane fuel cells: strain, ligand, and particle size effects. ACS Catal. 5, 176–186 (2015).
Vojislav R., Stamenkovic, N. M. M. Tailored high performance low-PGM alloy cathode catalysts (US DOE, 2017); https://www.hydrogen.energy.gov/pdfs/review17/fc140_stamenkovic_2017_o.pdf
Steinbach, A. J. Highly active, durable, and ultra-low PGM NSTF thin film ORR catalysts and supports (US DOE, 2017); https://www.hydrogen.energy.gov/pdfs/review17/fc143_steinbach_2017_o.pdf
Vojislav R. Stamenkovic, N. M. M. Tailored high performance low-PGM alloy cathode catalysts (US DOE 2016); https://www.hydrogen.energy.gov/pdfs/review16/fc140_stamenkovic_2016_o.pdf
Steinbach, A. J. Highly active, durable, and ultra-low PGM NSTF thin film ORR catalysts and supports. (US DOE, 2016); https://www.hydrogen.energy.gov/pdfs/review16/fc143_steinbach_2016_o.pdf
Park, Y.-C. et al. Investigation of the corrosion of carbon supports in polymer electrolyte fuel cells using simulated start-up/shutdown cycling. Electrochim. Acta 91, 195–207 (2013).
Ahluwalia, R. et al. Mechanism and kinetics of carbon corrosion in polymer electrolyte fuel cells during drive cycles. ECS Meet. Abstr. MA2015-02, 1503 (2015).
Borup, R. L. et al. Electrocatalyst layer degradation of PEM fuel cells. ECS Meet. Abstr. MA2014-02, 1201 (2014).
Chen, M. et al. Pt alloy nanoparticles decorated on large-size nitrogen-doped graphene tubes for highly stable oxygen-reduction catalysts. Nanoscale 10, 17318–17326 (2018).
Chong, L. et al. Ultralow-loading platinum-cobalt fuel cell catalysts derived from imidazolate frameworks. Science 362, 1276–1281 (2018).
Wang, X. X. et al. Ordered Pt3Co intermetallic nanoparticles derived from metal–organic frameworks for oxygen reduction. Nano Lett. 18, 4163–4171 (2018).
Zhang, H. et al. Single atomic iron catalysts for oxygen reduction in acidic media: particle size control and thermal activation. J. Am. Chem. Soc. 139, 14143 (2017). An atomically dispersed and nitrogen cordination single iron catalyst in the form of FeN 4 exhibited ever recorded ORR activity and stability in acidic media.
Chung, H. T. et al. Direct atomic-level insight into the active sites of a high-performance PGM-free ORR catalyst. Science 357, 479–484 (2017).
Miao, Z. et al. Atomically dispersed Fe-Nx/C electrocatalyst boosts oxygen catalysis via a new metal-organic polymer supramolecule strategy. Adv. Energy Mater. 8, 1801226 (2018).
Choi, J.-Y. et al. Is the rapid initial performance loss of Fe/N/C non precious metal catalysts due to micropore flooding? Energy Environ. Sci. 10, 296–305 (2017).
Wu, G., More, K. L., Johnston, C. M. & Zelenay, P. High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt. Science 332, 443–447 (2011).
Chokai, M., Daidou, T. & Nabae, Y. Development of Pt-free carbon-based catalyst for PEFC cathode prepared from polyacrylonitrile. ECS Trans. 64, 261–270 (2014).
Fu, X. et al. In situ polymer graphenization ingrained with nanoporosity in a nitrogenous electrocatalyst boosting the performance of polymer-electrolyte-membrane fuel cells. Adv. Mater. 29, 1604456 (2017).
Liang, H.-W., Wei, W., Wu, Z.-S., Feng, X. & Müllen, K. Mesoporous metal–nitrogen-doped carbon electrocatalysts for highly efficient oxygen reduction reaction. J. Am. Chem. Soc. 135, 16002–16005 (2013).
Wang, Y.-C. et al. S-doping of an Fe/N/C ORR catalyst for polymer electrolyte membrane fuel cells with high power density. Angew. Chem. Int. Ed. 54, 9907–9910 (2015).
Yang, L. et al. Unveiling the high-activity origin of single-atom iron catalysts for oxygen reduction reaction. PNAS 115, 6626–6631 (2018).
Sa, Y. J. et al. A general approach to preferential formation of active Fe-Nx sites in Fe-N/C electrocatalysts for efficient oxygen reduction reaction. J. Am. Chem. Soc. 138, 15046–15056 (2016).
Herranz, J. et al. Unveiling N-protonation and anion-binding effects on Fe/N/C catalysts for O2 reduction in proton-exchange-membrane fuel cells. J. Phys. Chem. C. 115, 16087–16097 (2011).
Lai, Q. et al. Metal-organic-framework-derived Fe-N/C electrocatalyst with five-coordinated Fe-Nx sites for advanced oxygen reduction in acid media. ACS Catal. 7, 1655–1663 (2017).
Zhang, C. et al. Networking pyrolyzed zeolitic imidazolate frameworks by carbon nanotubes improves conductivity and enhances oxygen-reduction performance in polymer-electrolyte-membrane fuel cells. Adv. Mater. 29, 1604556 (2017).
Myers, D. & Zelenay, P. Electrocat (electrocatalysis consortium) (US DOE, 2018); https://www.hydrogen.energy.gov/pdfs/review18/fc160_myers_2018_o.pdf (2018).
Litster, S. Advanced PGM-free cathode engineering for high power density and durability (US DOE, 2018); https://www.hydrogen.energy.gov/pdfs/review18/fc171_litster_2018_o.pdf (2018).
Wan, X. et al. Fe-N-C electrocatalyst with dense active sites and efficient mass transport for high-performance proton exchange membrane fuel cells. Nat. Catal. 2, 259–268 (2019).A Fe-N-C catalyst with unique concave and mesoporosity morphologies provides increased density of active sites and improved mass transfer.
Liu, K., Wu, G. & Wang, G. Role of local carbon structure surrounding FeN4 sites in boosting the catalytic activity for oxygen reduction. J. Phys. Chem. C. 121, 11319–11324 (2017).
Kneebone, J. L. et al. A combined probe-molecule, mössbauer, nuclear resonance vibrational spectroscopy, and density functional theory approach for evaluation of potential iron active sites in an oxygen reduction reaction catalyst. J. Phys. Chem. C. 121, 16283–16290 (2017).
You, B. et al. Bimetal-organic framework self-adjusted synthesis of support-free nonprecious electrocatalysts for efficient oxygen reduction. ACS Catal. 5, 7068–7076 (2015).
Yin, P. et al. Single cobalt atoms with precise N-coordination as superior oxygen reduction reaction catalysts. Angew. Chem. Int. Ed. 55, 10800–10805 (2016).
Wang, J. et al. Design of N-coordinated dual-metal sites: A stable and active Pt-free catalyst for acidic oxygen reduction reaction. J. Am. Chem. Soc. 139, 17281–17284 (2017).
Wang, X. X. et al. Nitrogen-coordinated single cobalt atom catalysts for oxygen reduction in proton exchange membrane fuel cells. Adv. Mater. 30, 1706758 (2018). An atomically dispersed and nitrogen-coordinated Co site catalyst derived from Co-doped ZIF-8 demonstrated promising performance in PEMFCs.
He, Y. et al. Highly active atomically dispersed CoN4 fuel cell cathode catalysts derived from surfactant-assisted MOFs: carbon-shell confinement strategy. Energy Environ. Sci. 12, 250–260 (2019).
Xiao, M. et al. Identification of binuclear Co2N5 active sites for oxygen reduction reaction with more than one magnitude higher activity than single atom CoN4 site. Nano Energy 46, 396–403 (2018).
Wang, X. et al. Size-controlled large-diameter and few-walled carbon nanotube catalysts for oxygen reduction. Nanoscale 7, 20290–20298 (2015).
Gupta, S. et al. Quaternary FeCoNiMn-based nanocarbon electrocatalysts for bifunctional oxygen reduction and evolution: promotional role of Mn doping in stabilizing carbon. ACS Catal. 7, 8386–8393 (2017).
Zhong, Y. et al. The constraints of transition metal substitutions (Ti, Cr, Mn, Co and Ni) in magnetite on its catalytic activity in heterogeneous fenton and UV/Fenton reaction: From the perspective of hydroxyl radical generation. Appl. Catal. B 150–151, 612–618 (2014).
Li, J. et al. Atomically dispersed manganese catalysts for oxygen reduction in proton-exchange membrane fuel cells. Nat. Catal. 1, 935–945 (2018). Astudy on atomically dispersed and nitrogen-coordinated Mn sites demonstrated a concept that Mn-based catalysts can minimize the possible Fenton-realted reactions in PEMFCs and thus eventually resolve the stability issues of PGM-free catalysts.
Nallathambi, V., Lee, J.-W., Kumaraguru, S. P., Wu, G. & Popov, B. N. Development of high performance carbon composite catalyst for oxygen reduction reaction in PEM proton exchange membrane fuel cells. J. Power Sources 183, 34–42 (2008).
Shao, Y., Dodelet, J.-P., Wu, G. & Zelenay, P. PGM-free cathode catalysts for PEM fuel cells: a mini-review on stability challenges. Adv. Mater. 31, 1807615 (2019). A short summary of stability challenges of current PGM-free cathode catalysts in PEMFCs and possible solutions to address them.
Chong, L. et al. Investigation of oxygen reduction activity of catalysts derived from Co and Co/Zn methyl-imidazolate frameworks in proton exchange membrane fuel cells. ChemElectroChem 3, 1541–1545 (2016).
Choi, C. H. et al. Stability of Fe-N-C catalysts in acidic medium studied by operando spectroscopy. Angew. Chem. Int. Ed. 54, 12753–12757 (2015).
Chenitz, R. et al. A specific demetalation of Fe-N4 catalytic sites in the micropores of NC_Ar + NH3 is at the origin of the initial activity loss of the highly active Fe/N/C catalyst used for the reduction of oxygen in PEM fuel cells. Energy Environ. Sci. 11, 365–382 (2018).
Ferrandon, M. et al. Stability of iron species in heat-treated polyaniline-iron-carbon polymer electrolyte fuel cell cathode catalysts. Electrochim. Acta 110, 282–291 (2013).
Wu, G. et al. Synthesis of nitrogen-doped onion-like carbon and its use in carbon-based CoFe binary non-precious-metal catalysts for oxygen-reduction. Carbon 49, 3972–3982 (2011).
Komini Babu, S., Chung, H. T., Zelenay, P. & Litster, S. Resolving electrode morphology’s impact on platinum group metal-free cathode performance using nano-CT of 3D hierarchical pore and ionomer distribution. ACS Appl. Mater. Interfaces 8, 32764–32777 (2016).
Acknowledgements
We acknowledge the financial support from US Department of Energy, Office of Energy Efficiency and Renewable Energy (EERE), Fuel Cell Technology Office (DE-EE0008075, DE-EE0008076, and DE-EE0008417) along with National Science Foundation (CBET-1604392, 1804326).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, X.X., Swihart, M.T. & Wu, G. Achievements, challenges and perspectives on cathode catalysts in proton exchange membrane fuel cells for transportation. Nat Catal 2, 578–589 (2019). https://doi.org/10.1038/s41929-019-0304-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-019-0304-9
This article is cited by
-
Machine-learning-accelerated design of high-performance platinum intermetallic nanoparticle fuel cell catalysts
Nature Communications (2024)
-
Identifying the distinct roles of dual dopants in stabilizing the platinum-nickel nanowire catalyst for durable fuel cell
Nature Communications (2024)
-
Rational design of superior catalysts from topological semimetals with nontrivial energy window
Rare Metals (2024)
-
The reformation of catalyst: From a trial-and-error synthesis to rational design
Nano Research (2024)
-
Electronegativity-assisted optimized electronic structure of functionalized-Pt catalysts for boosting oxygen reduction kinetics
Rare Metals (2024)