Abstract
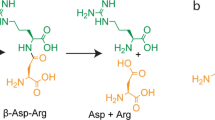
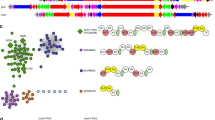
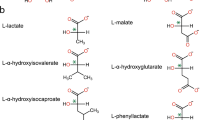
Vitamin K-dependent carboxylase (VKDC) enzymes modify glutamate residues in mammalian vitamin K-dependent proteins, generating γ-carboxyglutamic acids with malonate moieties that mediate important physiological responses such as blood coagulation. Proteins with sequence similarity to mammalian VKDC are also found in bacteria; however, their function remains unknown. The antibiotic malonomycin from Streptomyces rimosus contains an unusual malonate group, of unknown origin, that is essential for its biological activity. Here, we show that a bacterial VKDC orthologue (MloH) is responsible for the malonic acid moiety in malonomycin. Using CRISPR/Cas9 gene editing, complementation and mutagenesis experiments, this VKDC-like enzyme was shown to α-carboxylate an aspartyl residue within a hybrid polyketide–nonribosomal peptide intermediate during malonomycin biosynthesis. This study reveals a highly unusual biosynthetic pathway to malonic acid-containing metabolites, providing a functional role for VKDC-like proteins in prokaryotes and a vitamin K-dependent carboxylation reaction with a non-proteinogenic substrate.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request. Nucleotide sequences for the malonomycin BGCs are deposited in GenBank (accession numbers MH104948 (S. rimosus paramyceticus R2374) and MH104947 (S. rimosus paromomycinus NRRL 2455). The whole genome shotgun sequence for N. gamkensis NBRC 108242, used for the identification of the malonomycin-like cluster from this strain, was obtained from GenBank National Center for Biotechnology Information reference sequence NZ_BDBM01000044.1. Details on nucleotide sequences and proposed annotations are detailed in the Supplementary Information.
References
Batelaan, J. G., Barnick, J. W. F. K., van der Baan, J. L. & Bickelhaupt, F. The structure of the antibiotic K16. I. The dipeptide side chain. Tetrahedron Lett. 13, 3103–3106 (1972).
Batelaan, J. G., Barnick, J. W. F. K., van der Baan, J. L. & Bickelhaupt, F. The structure of the antibiotic K16. II. Chromophore and total structure. Tetrahedron Lett. 13, 3107–3110 (1972).
Schipper, D., van der Baan, J. L. & Bickelhaupt, F. Biosynthesis of malonomicin. Part 1. 13C nuclear magnetic resonance spectrum and feeding experiments with 13C-labelled precursors. J. Chem. Soc. Perkin Trans. 10, 2017–2022 (1979).
Chan, Y. A., Podevels, A. M., Kevany, B. M. & Thomas, M. G. Biosynthesis of polyketide synthase extender units. Nat. Prod. Rep. 26, 90–114 (2009).
Stenflo, J., Fernlund, P., Egan, W. & Roepstorff, P. Vitamin K dependent modifications of glutamic acid residues in prothrombin. Proc. Natl Acad. Sci. USA 71, 2730–2733 (1974).
Esmon, C. T., Sadowski, J. A. & Suttie, J. W. A new carboxylation reaction. J. Biol. Chem. 250, 4744–4748 (1975).
Wu, S.-M., Morris, D. P. & Stafford, D. W. Identification and purification to near homogeneity of the vitamin K-dependent carboxylase. Proc. Natl Acad. Sci. USA 88, 2236–2240 (1991).
Wu, S.-M., Cheung, W.-F., Frazier, D. & Stafford, D. W. Cloning and expression of the cDNA for human gamma-glutamyl carboxylase. Science 254, 1634–1636 (1991).
Dowd, P., Ham, S. W. & Geib, S. J. Mechanism of action of vitamin K. J. Am. Chem. Soc. 113, 7734–7743 (1991).
Dowd, P., Ham, S. W. & Hershline, R. Role of oxygen in the vitamin K-dependent carboxylation reaction: incorporation of a second atom of 18O from molecular oxygen-18O2 into vitamin K oxide during carboxylase activity. J. Am. Chem. Soc. 114, 7613–7617 (1992).
Dowd, P., Hershline, R., Ham, S. W. & Naganathan, S. Vitamin K and energy transduction: a base strength amplification mechanism. Science 269, 1684–1691 (1995).
Fasco, M. J., Preusch, P. C., Hildebrandt, E. & Suttie, J. W. Formation of hydroxyvitamin K by vitamin K epoxide reductase of warfarin-resistant rats. J. Biol. Chem. 258, 4372–4380 (1983).
Bell, R. G. & Matschiner, J. T. Warfarin and the inhibition of vitamin K activity by an oxide metabolite. Nature 237, 32–33 (1972).
Rishavy, M. A. et al. The vitamin K-dependent carboxylase has been acquired by Leptospira pathogens and shows altered activity that suggests a role other than protein carboxylation. J. Biol. Chem. 280, 34870–34877 (2005).
Kobylarz, M. J. et al. Synthesis of l-2,3-diaminopropionic acid, a siderophore and antibiotic precursor. Chem. Biol. 21, 379–388 (2014).
Rishavy, M. A. et al. Brønsted analysis reveals Lys218 as the carboxylase active site base that deprotonates vitamin K hydroquinone to initiate vitamin K-dependent protein carboxylation. Biochemistry 45, 13239–13248 (2006).
Rishavy, M. A. & Berkner, K. L. Insight into the coupling mechanism of the vitamin K-dependent carboxylase: mutation of histidine 160 disrupts glutamic acid carbanion formation and efficient coupling of vitamin K epoxidation to glutamic acid carboxylation. Biochemistry 47, 9836–9846 (2008).
Alderson, G., Goodfellow, M. & Minnikin, D. E. Menaquinone composition in the classification of Streptomyces and other sporoactinomycetes. Microbiology 131, 1671–1679 (1985).
Hiratsuka, T. et al. An alternative menaquinone biosynthetic pathway operating in microorganisms. Science 321, 1670–1673 (2008).
Nowicka, B. & Kruk, J. Occurrence, biosynthesis and function of isoprenoid quinones. Biochim. Biophys. Acta 1797, 1587–1605 (2010).
Schultz, J. HTTM, a horizontally transferred transmembrane domain. Trends Biochem. Sci. 29, 4–7 (2004).
Cobb, R. E., Wang, Y. & Zhao, H. High-efficiency multiplex genome editing of Streptomyces species using an engineered CRISPR/Cas system. ACS Synth. Biol. 4, 723–728 (2015).
Davidsen, J. M., Bartley, D. M. & Townsend, C. A. Nonribosomal propeptide precursor in nocardicin A biosynthesis predicted from adenylation domain specificity dependent on the MbtH family protein NocI. J. Am. Chem. Soc. 135, 1749–1759 (2013).
Reimer, D., Pos, K. M., Thines, M., Grün, P. & Bode, H. B. A natural prodrug activation mechanism in nonribosomal peptide synthesis. Nat. Chem. Biol. 7, 888–890 (2011).
Luo, Y. et al. Validation of the intact zwittermicin A biosynthetic gene cluster and discovery of a complementary resistance mechanism in Bacillus thuringiensis. Antimicrob. Agents Chemother. 55, 4161–4169 (2011).
Marahiel, M. A., Stachelhaus, T. & Mootz, H. D. Modular peptide synthetases involved in nonribosomal peptide synthesis. Chem. Rev. 97, 2651–2673 (1997).
Bergendahl, V., Linne, U. & Marahiel, M. A. Mutational analysis of the C-domain in nonribosomal peptide synthesis. Eur. J. Biochem. 269, 620–629 (2002).
Webb, M. R. A continuous spectrophotometric assay for inorganic phosphate and for measuring phosphate release kinetics in biological systems. Proc. Natl Acad. Sci. USA 89, 4884–4887 (1992).
Pavela-Vrancic, M., Dieckmann, R. & Von Döhren, H. ATPase activity of non-ribosomal peptide synthetases. Biochim. Biophys. Acta 1696, 83–91 (2004).
Stachelhaus, T., Mootz, H. D. & Marahiel, M. A. The specificity-conferring code of adenylation domains in nonribosomal peptide synthetases. Chem. Biol. 6, 493–505 (1999).
Szókán, G., Mezö, G. & Hudecz, F. Application of Marfey’s reagent in racemization studies of amino acids and peptides. J. Chromatogr. A 444, 115–122 (1988).
Bochmann, S. M., Spieß, T., Kötter, P. & Entian, K. D. Synthesis and succinylation of subtilin-like lantibiotics are strongly influenced by glucose and transition state regulator AbrB. Appl. Environ. Microbiol. 81, 614–622 (2015).
Cochrane, S. A., Surgenor, R. R., Khey, K. M. W. & Vederas, J. C. Total synthesis and stereochemical assignment of the antimicrobial lipopeptide cerexin A1. Org. Lett. 17, 5428–5431 (2015).
Spieß, T., Korn, S. M., Kötter, P. & Entian, K. D. Activation of histidine kinase SpaK is mediated by the N-terminal portion of subtilin-like lantibiotics and is independent of lipid II. Appl. Environ. Microbiol. 81, 5335–5343 (2015).
Chan, W. C., Bycroft, B. W., Leyland, M. L., Lian, L.-Y. & Roberts, G. C. K. A novel post-translational modification of the peptide antibiotic subtilin: isolation and characterization of a natural variant from Bacillus subtilis A.T.C.C. 6633. Biochem. J. 291, 23–27 (1993).
Altschul, S. F. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 (1997).
Rutherford, K. et al. Artemis: sequence visualization and annotation. Bioinformatics 16, 944–945 (2000).
Acknowledgements
We thank BBSRC (grant BB/K002341/1) and Syngenta for funding. The SYNBIOCHEM Centre (grant BB/M017702/1) and Michael Barber Centre for Mass Spectrometry at the University of Manchester provided access to mass spectrometry instrumentation. J. Vincent and N. Mulholland (Syngenta) are also acknowledged for helpful discussion.
Author information
Authors and Affiliations
Contributions
B.J.C.L., Y. Zhuo, M.W. and J.M. designed the experiments. B.J.C.L., Y.Z., M.W., D.F., Y. Zhang, A.M. and L.R. carried out the experiments. M.S. and P.F.L. sequenced and annotated the genomes. B.J.C.L. and J.M. wrote the manuscript. All authors analysed the data and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Figs 1–16, Supplementary Tables 1–5, Supplementary References
Rights and permissions
About this article
Cite this article
Law, B.J.C., Zhuo, Y., Winn, M. et al. A vitamin K-dependent carboxylase orthologue is involved in antibiotic biosynthesis. Nat Catal 1, 977–984 (2018). https://doi.org/10.1038/s41929-018-0178-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-018-0178-2
This article is cited by
-
Genome mining reveals novel biosynthetic gene clusters in entomopathogenic bacteria
Scientific Reports (2023)
-
Commensal production of a broad-spectrum and short-lived antimicrobial peptide polyene eliminates nasal Staphylococcus aureus
Nature Microbiology (2023)
-
CRISPR-Cas system: a potential alternative tool to cope antibiotic resistance
Antimicrobial Resistance & Infection Control (2020)
-
Pathway to malonic acid-containing metabolites
Nature Catalysis (2018)