Abstract
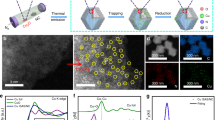
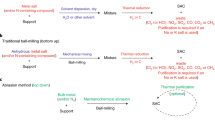
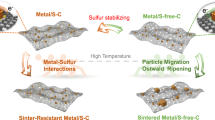
Single-atom catalysts exhibit intriguing properties and receive widespread interest for their effectiveness in promoting a variety of catalytic reactions, making them highly desired motifs in materials science. However, common approaches to the synthesis of these materials often require tedious procedures and lack appropriate interactions between the metal atoms and supports. Here, we report a simple and practical strategy to access the large-scale synthesis of single-atom catalysts via direct atoms emitting from bulk metals, and the subsequent trapping on nitrogen-rich porous carbon with the assistance of ammonia. First, the ammonia coordinates with the copper atoms to form volatile Cu(NH3)x species based on the strong Lewis acid–base interaction. Then, following transportation under an ammonia atmosphere, the Cu(NH3)x species are trapped by the defects on the nitrogen-rich carbon support, forming the isolated copper sites. This strategy is readily scalable and has been confirmed as feasible for producing functional single-atom catalysts at industrial levels.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The main data supporting the findings of this study are available within the article and its Supplementary Information. Extra data are available from the corresponding authors upon request.
References
Boronat, M. et al. Theoretical and experimental insights into the origin of the catalytic activity of subnanometric gold clusters: attempts to predict reactivity with clusters and nanoparticles of gold. Acc. Chem. Res. 47, 834–844 (2013).
Wei, H. et al. FeOx-supported platinum single-atom and pseudo-single-atom catalysts for chemoselective hydrogenation of functionalized nitroarenes. Nat. Commun. 5, 5634 (2014).
Yan, H. et al. Single-atom Pd1/graphene catalyst achieved by atomic layer deposition: remarkable performance in selective hydrogenation of 1,3-butadiene. J. Am. Chem. Soc. 137, 10484–10487 (2015).
Nie, L. et al. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation. Science 358, 1419–1423 (2017).
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011).
Kwon, Y. et al. Selective activation of methane on single-atom catalyst of rhodium dispersed on zirconia for direct conversion. J. Am. Chem. Soc. 139, 17694–17699 (2017).
Guo, X. et al. Direct, nonoxidative conversion of methane to ethylene, aromatics, and hydrogen. Science 344, 616–619 (2014).
Yin, P. et al. Single cobalt atoms with precise N-coordination as superior oxygen reduction reaction catalysts. Angew. Chem. Int. Ed. 55, 10800–10805 (2016).
Chung, H. T. et al. Direct atomic-level insight into the active sites of a high-performance PGM-free ORR catalyst. Science 357, 479–484 (2017).
Chen, P. et al. Atomically dispersed iron–nitrogen species as electrocatalysts for bifunctional oxygen evolution and reduction reactions. Angew. Chem. Int. Ed. 56, 610–614 (2017).
Zheng, Y. et al. Molecule-level g-C3N4 coordinated transition metals as a new class of electrocatalysts for oxygen electrode reactions. J. Am. Chem. Soc. 139, 3336–3339 (2017).
Zhang, L. et al. Efficient and durable Au alloyed Pd single-atom catalyst for the Ullmann reaction of aryl chlorides in water. ACS Catal. 4, 1546–1553 (2014).
Zhang, X. et al. Catalytically active single-atom niobium in graphitic layers. Nat. Commun. 4, 1924 (2013).
Jiao, Y. et al. Molecular scaffolding strategy with synergistic active centers to facilitate electrocatalytic CO2 reduction to hydrocarbon/alcohol. J. Am. Chem. Soc. 139, 18093–18100 (2017).
Zhang, L., Han, L., Liu, H., Liu, X. & Luo, J. Potential-cycling synthesis of single platinum atoms for efficient hydrogen evolution in neutral media. Angew. Chem. Int. Ed. 56, 13694–13698 (2017).
Long, R. et al. Isolation of Cu atoms in Pd lattice: forming highly selective sites for photocatalytic conversion of CO2 to CH4. J. Am. Chem. Soc. 139, 4486–4492 (2017).
Abbet, S. et al. Acetylene cyclotrimerization on supported size-selected Pdn clusters (1 ≤ n ≤ 30): one atom is enough! J. Am. Chem. Soc. 122, 3453–3457 (2000).
Peters, A. W. et al. Atomically precise growth of catalytically active cobalt sulfide on flat surfaces and within a metal-organic framework via atomic layer deposition. ACS Nano 9, 8484–8490 (2015).
Lin, J. et al. Remarkable performance of Ir1/FeOx single-atom catalyst in water gas shift reaction. J. Am. Chem. Soc. 135, 15314–15317 (2013).
Lin, L. et al. Low-temperature hydrogen production from water and methanol using Pt/α-MoC catalysts. Nature 544, 80–83 (2017).
Liu, P. et al. Photochemical route for synthesizing atomically dispersed palladium catalysts. Science 352, 797–800 (2016).
Jones, J. et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 353, 150–154 (2016).
Chen, S. et al. Three-dimensional N-doped graphene hydrogel/NiCo double hydroxide electrocatalysts for highly efficient oxygen evolution. Angew. Chem. Int. Ed. 52, 13567–13570 (2013).
Qu, L. et al. Nitrogen-doped graphene as efficient metal-free electrocatalyst for oxygen reduction in fuel cells. ACS Nano 4, 1321–1326 (2010).
Gong, J. et al. Synthesis of ethanol via syngas on Cu/SiO2 catalysts with balanced Cu0–Cu+ sites. J. Am. Chem. Soc. 134, 13922–13925 (2012).
Wang, J. et al. Design of N-coordinated dual-metal sites: a stable and active Pt-free catalyst for acidic oxygen reduction reaction. J. Am. Chem. Soc. 139, 17281–17284 (2017).
Nesselberger, M. et al. The particle size effect on the oxygen reduction reaction activity of Pt catalysts: influence of electrolyte and relation to single crystal models. J. Am. Chem. Soc. 133, 17428–17433 (2011).
Suntivich, J. et al. Electrocatalytic measurement methodology of oxide catalysts using a thin-film rotating disk electrode. J. Electrochem. Soc. 157, B1263–B1268 (2010).
Piana, M., Catanorchi, S. & Gasteiger, H. Kinetics of non-platinum group metal catalysts for the oxygen reduction reaction in alkaline medium. ECS Trans. 16, 2045–2055 (2008).
Lu, Y. et al. Strongly coupled Pd nanotetrahedron/tungsten oxide nanosheet hybrids with enhanced catalytic activity and stability as oxygen reduction electrocatalysts. J. Am. Chem. Soc. 136, 11687–11697 (2014).
Choi, C. et al. Unraveling the nature of sites active toward hydrogen peroxide reduction in Fe–N–C catalysts. Angew. Chem. Int. Ed. 129, 8935–8938 (2017).
Zeradjanin, A. et al. Frequent pitfalls in the characterization of electrodes designed for electrochemical energy conversion and storage. ChemSusChem. 11, 1278–1284 (2018).
Ramaswamy, N. & Mukerjee, S. Fundamental mechanistic understanding of electrocatalysis of oxygen reduction on Pt and non-Pt surfaces: acid versus alkaline media. Adv. Phys. Chem. 2012, 491604 (2012).
Ramaswamy, N. et al. Activity descriptor identification for oxygen reduction on nonprecious electrocatalysts: linking surface science to coordination chemistry. J. Am. Chem. Soc. 135, 15443–15449 (2013).
Liang, Y. et al. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 10, 780–786 (2011).
Acknowledgements
This work was supported by the National Key R&D Program of China 2017YFA (0208300) and National Natural Science Foundation of China (21522107, 21671180, 21521091, 21390393 and U1463202). We thank the photoemission endstations beamline 1W1B station in the Beijing Synchrotron Radiation Facility (BSRF), BL14W1 at the Shanghai Synchrotron Radiation Facility and BL10B and BL11U at the National Synchrotron Radiation Laboratory for help with the characterizations.
Author information
Authors and Affiliations
Contributions
Y.Li and Y.W. conceived the idea and co-wrote the paper. Y.Q. carried out the sample synthesis, characterization and ORR measurement. Z.L., Z.Z., Z.Y., C.Z., T.Y., X.W., F.Z., C.Z. and J.W. helped with the modification of the paper. W.C. helped with the XAFS measurements and discussion. Y.Lin helped with the spherical aberration electron microscopy tests and discussion. All authors contributed to the overall scientific interpretation and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–27 & Supplementary Tables 1–3
Rights and permissions
About this article
Cite this article
Qu, Y., Li, Z., Chen, W. et al. Direct transformation of bulk copper into copper single sites via emitting and trapping of atoms. Nat Catal 1, 781–786 (2018). https://doi.org/10.1038/s41929-018-0146-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-018-0146-x
This article is cited by
-
In situ modulating coordination fields of single-atom cobalt catalyst for enhanced oxygen reduction reaction
Nature Communications (2024)
-
Sequential co-reduction of nitrate and carbon dioxide enables selective urea electrosynthesis
Nature Communications (2024)
-
Single-atomic activation on ZnIn2S4 basal planes boosts photocatalytic hydrogen evolution
Nano Research (2024)
-
Free-Standing Single-Atom Catalyst-Based Electrodes for CO2 Reduction
Electrochemical Energy Reviews (2024)
-
Graphene-anchored sodium single atoms: A highly active and stable catalyst for transesterification reaction
Nano Research (2024)