Abstract
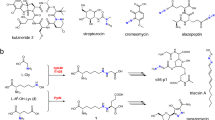
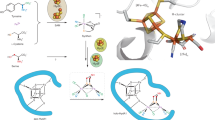
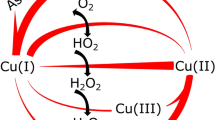
A structural unit found in the active site of some copper proteins, the histidine brace, is comprised of an N-terminal histidine that chelates a single copper ion through its amino terminus NH2 and the π–N of its imidazole side chain. Coordination is completed by the τ-N of a further histidine side chain, to give an overall N3 T-shaped coordination at the copper ion. The histidine brace appears in several proteins, including lytic polysaccharide monooxygenases LPMOs and particulate methane monooxygenases pMMOs, both of which catalyse the oxidation of substrates with strong C–H bonds (bond dissociation enthalpies ~100 kcal mol–1). As such, the copper histidine brace is the focus of research aimed at understanding how nature catalyses the oxidation of unactivated C–H bonds. In this Perspective, we evaluate these studies, which further give bioinspired direction to coordination chemists in the design and preparation of small molecule copper oxidation catalysts.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Quinlan, R. J. et al. Insights into the oxidative degradation of cellulose by a copper metalloenzyme that exploits biomass components. Proc. Natl Acad. Sci. USA 108, 15079–15084 (2011).
Phillips, C. M., Beeson, W. T., Cate, J. H. & Marletta, M. A. Cellobiose dehydrogenase and a copper-dependent polysaccharide monooxygenase potentiate cellulose degradation by Neurospora crassa. ACS Chem. Biol. 6, 1399–1406 (2011).
Zhang, L. et al. Intermolecular transfer of copper ions from the CopC protein of pseudomonas syringae. Crystal structures of fully loaded CuICuII forms. J. Am. Chem. Soc. 128, 5834–5850 (2006).
Vaaje-Kolstad, G. et al. An oxidative enzyme boosting the enzymatic conversion of recalcitrant polysaccharides. Science 330, 219–222 (2010).
Hemsworth, G. R., Davies, G. J. & Walton, P. H. Recent insights into copper-containing lytic polysaccharide mono-oxygenases. Curr. Opin. Struct. Biol. 23, 660–668 (2013).
Walton, P. H. & Davies, G. J. On the catalytic mechanisms of lytic polysaccharide monooxygenases. Curr. Opin. Chem. Biol. 31, 195–207 (2016).
Gao, J., Thomas, D. A., Sohn, C. H. & Beauchamp, J. L. Biomimetic reagents for the selective free radical and acid–base chemistry of glycans: application to glycan structure determination by mass spectrometry. J. Am. Chem. Soc. 135, 10684–10692 (2013).
Vu, V. V. & Ngo, S. T. Copper active site in polysaccharide monooxygenases. Coord. Chem. Rev. 368, 134–157 (2018).
Lieberman, R. L. & Rosenzweig, A. C. Crystal structure of a membrane-bound metalloenzyme that catalyses the biological oxidation of methane. Nature 434, 177–182 (2005).
Luo, Y.-R. Comprehensive Handbook of Chemical Bond Energies (CRC, 2007).
Solomon, E. I. et al. Copper active sites in biology. Chem. Rev. 114, 3659–3853 (2014).
Vaaje-Kolstad, G. et al. The non-catalytic chitin-binding protein CBP21 from Serratia marcescens is essential for chitin degradation. J. Biol. Chem. 280, 28492–28497 (2005).
Vaaje-Kolstad, G. et al. Crystal structure and binding properties of the Serratia marcescens chitin-binding Protein CBP21. J. Biol. Chem. 280, 11313–11319 (2005).
Harris, P. V. et al. Stimulation of lignocellulosic biomass hydrolysis by proteins of glycoside hydrolase family 61: structure and function of a large, enigmatic family. Biochemistry 49, 3305–3316 (2010).
Karkehabadi, S. et al. The first structure of a glycoside hydrolase family 61 member, Cel61B from hypocrea jecorina, at 1.6 Å resolution. J. Mol. Biol. 383, 144–154 (2008).
Hemsworth, G. R., Johnston, E. M., Davies, G. J. & Walton, P. H. Lytic polysaccharide monooxygenases in biomass conversion. Trends Biotechnol. 33, 747–761 (2015).
Levasseur, A. et al. Expansion of the enzymatic repertoire of the CAZy database to integrate auxiliary redox enzymes. Biotechnol. Biofuels 6, 41 (2013).
Hemsworth, G. R., Henrissat, B., Davies, G. J. & Walton, P. H. Discovery of a new family of lytic polysaccharide mono-oxygenases. Nat. Chem. Biol. 10, 122–126 (2014).
Vu, V. V. et al. A family of starch-active polysaccharide monooxygenases. Proc. Natl Acad. Sci. USA 111, 13822–13827 (2014).
Lo Leggio, L. et al. Structure and boosting activity of a starch-degrading lytic polysaccharide monooxygenase. Nat. Commun. 6, 5961 (2015).
Couturier, M. et al. Lytic xylan oxidases from wood-decay fungi unlock biomass degradation. Nat. Chem. Biol. 14, 306–310 (2018).
Sabbadin, F. et al. An ancient family of lytic polysaccharide monooxygenases with roles in arthropod development and biomass digestion. Nat. Commun. 9, 756 (2018).
Meier, K. K. et al. Oxygen activation by Cu LPMOs in recalcitrant carbohydrate polysaccharide conversion to monomer sugars. Chem. Rev. 118, 2593–2635 (2017).
Vaaje-Kolstad, G. et al. Structural diversity of lytic polysaccharide monooxygenases. Curr. Opin. Struct. Biol. 44, 67–76 (2017).
Span, E. A. et al. The role of the secondary coordination ssphere in a fungal polysaccharide monooxygenase. ACS Chem. Biol. 12, 1095–1103 (2017).
Mann, S. I., Heinisch, T., Ward, T. R. & Borovik, A. S. Peroxide activation regulated by hydrogen bonds within artificial Cu proteins. J. Am. Chem. Soc. 139, 17289–17292 (2017).
Hemsworth, G. R. The copper active site of CBM33 polysaccharide oxygenases. J. Am. Chem. Soc. 135, 6069–6077 (2013).
Forsberg, Z. et al. Structural determinants of bacterial lytic polysaccharide monooxygenase functionality. J. Biol. Chem. 293, 1397–1412 (2018).
Frandsen, K. E. H. et al. The molecular basis of polysaccharide cleavage by lytic polysaccharide monooxygenases. Nat. Chem. Biol. 12, 298–303 (2016).
Evans, J. P., Ahn, K. & Klinman, J. P. Evidence that dioxygen and substrate activation are tightly coupled in dopamine β-monooxygenase: implications for the reactive oxygen species. J. Biol. Chem. 278, 49691–49698 (2003).
Sevrioukova, I. F. & Poulos, T. L. Understanding the mechanism of cytochrome P450 3A4: recent advances and remaining problems. Dalton Trans. 42, 3116–3126 (2013).
Gudmundsson, M. et al. Structural and electronic snapshots during the transition from a Cu(ii) to Cu(i) metal center of a lytic polysaccharide monooxygenase by X-ray photoreduction. J. Biol. Chem. 289, 18782–18792 (2014).
Hedegard, E. D. & Ryde, U. Molecular mechanism of lytic polysaccharide monooxygenases. Chem. Sci. 9, 3866–3880 (2018).
Kjaergaard, C. H. et al. Spectroscopic and computational insight into the activation of O2 by the mononuclear Cu center in polysaccharide monooxygenases. Proc. Natl Acad. Sci. USA 111, 8797–8802 (2014).
Li, X. et al. Structural basis for substrate targeting and catalysis by fungal polysaccharide monooxygenases. Structure 20, 1051–1061 (2012).
McDonald, M. R., Fredericks, F. C. & Margerum, D. W. Characterization of copper(iii)−tetrapeptide complexes with histidine as the third residue. Inorg. Chem. 36, 3119–3124 (1997).
Bacik, J.-P. et al. Neutron and atomic resolution X-ray structures of a lytic polysaccharide monooxygenase reveal copper-mediated dioxygen binding and evidence for N-terminal deprotonation. Biochemistry 56, 2529–2532 (2017).
Balasubramanian, R. et al. Oxidation of methane by a biological dicopper centre. Nature 465, 115–119 (2010).
Lee, J. Y. & Karlin, K. D. Elaboration of copper-oxygen mediated C–H activation chemistry in consideration of future fuel and feedstock generation. Curr. Opin. Chem. Biol. 25, 184–193 (2015).
Wang, V. C. C. et al. Alkane oxidation: methane monooxygenases, related enzymes, and their biomimetics. Chem. Rev. 117, 8574–8621 (2017).
Sommerhalter, M., Lieberman, R. L. & Rosenzweig, A. C. X-ray crystallography and biological metal centers: is seeing believing? Inorg. Chem. 44, 770–778 (2005).
Lieberman, R. L. et al. Characterization of the particulate methane monooxygenase metal centers in multiple redox states by X-ray absorption spectroscopy. Inorg. Chem. 45, 8372–8381 (2006).
Culpepper, M. A. et al. Identification of the valence and coordination environment of the particulate methane monooxygenase copper centers by advanced EPR characterization. J. Am. Chem. Soc. 136, 11767–11775 (2014).
Culpepper, M. A., Cutsail, G. E., Hoffman, B. M. & Rosenzweig, A. C. Evidence for oxygen binding at the active site of particulate methane monooxygenase. J. Am. Chem. Soc. 134, 7640–7643 (2012).
Itoyama, S. et al. Possible peroxo state of the dicopper site of particulate methane monooxygenase from combined quantum mechanics and molecular mechanics calculations. Inorg. Chem. 55, 2771–2775 (2016).
Yumura, T. et al. Roles of water molecules in modulating the reactivity of dioxygen-bound Cu-ZSM-5 toward methane: a theoretical prediction. ACS Catalysis 6, 2487–2495 (2016).
Yoshizawa, K. & Shiota, Y. Conversion of methane to methanol at the mononuclear and dinuclear copper sites of particulate methane monooxygenase (pMMO): a DFT and QM/MM study. J. Am. Chem. Soc. 128, 9873–9881 (2006).
Citek, C., Herres-Pawlis, S. & Stack, T. D. P. Low temperature syntheses and reactivity of Cu2O2 active-site models. Acc. Chem. Res. 48, 2424–2433 (2015).
Elwell, C. E. et al. Copper–oxygen complexes revisited: structures, spectroscopy, and reactivity. Chem. Rev. 117, 2059–2107 (2017).
Smith, S. M. et al. Crystal structure and characterization of particulate methane monooxygenase from methylocystis species strain M. Biochemistry 50, 10231–10240 (2011).
Cao, L., Caldararu, O., Rosenzweig, A. C. & Ryde, U. Quantum refinement does not support dinuclear copper sites in crystal structures of particulate methane monooxygenase. Angew. Chem. Int. Ed. 57, 162–166 (2018).
Ro, S. Y. et al. From micelles to bicelles: effect of the membrane on particulate methane monooxygenase activity. J. Biol. Chem. https://doi.org/10.1074/jbc.RA1118.003348 (2018).
Allen, S. E., Walvoord, R. R., Padilla-Salinas, R. & Kozlowski, M. C. Aerobic copper-catalyzed organic reactions. Chem. Rev. 113, 6234–6458 (2013).
Bissaro, B. et al. Oxidative cleavage of polysaccharides by monocopper enzymes depends on H2O2. Nat. Chem. Biol. 13, 1123–1128 (2017).
Hangasky, J. A., Iavarone, A. T. & Marletta, M. A. Reactivity of O2 versus H2O2 with polysaccharide monooxygenases. Proc. Natl Acad. Sci. USA 115, 4915–4920 (2018).
Kim, S. et al. Amine oxidative N-dealkylation via cupric hydroperoxide Cu–OOH homolytic cleavage followed by site-specific fenton chemistry. J. Am. Chem. Soc. 137, 2867–2874 (2015).
Mirica, L. M., Ottenwaelder, X. & Stack, T. D. P. Structure and spectroscopy of copper−dioxygen complexes. Chem. Rev. 104, 1013–1046 (2004).
Lewis, E. A. & Tolman, W. B. Reactivity of dioxygen−copper systems. Chem. Rev. 104, 1047–1076 (2004).
Liu, J. J., Diaz, D. E., Quist, D. A. & Karlin, K. D. Copper(i)–dioxygen adducts and copper enzyme mechanisms. Isr. J. Chem. 56, 9–10 (2016).
Quist, D. A., Diaz, D. E., Liu, J. J. & Karlin, K. D. Activation of dioxygen by copper metalloproteins and insights from model complexes. J. Biol. Inorg. Chem. 22, 253–288 (2017).
Cramer, C. J. & Tolman, W. B. Mononuclear Cu–O2 complexes: geometries, spectroscopic properties, electronic structures, and reactivity. Acc. Chem. Res. 40, 601–608 (2007).
Itoh, S. Developing mononuclear copper-active-oxygen complexes relevant to reactive intermediates of biological oxidation reactions. Acc. Chem. Res. 48, 2066–2074 (2015).
Gagnon, N. & Tolman, W. B. [CuO]+ and [CuOH]2+ complexes: intermediates in oxidation catalysis? Acc. Chem. Res. 48, 2126–2131 (2015).
Iovan, D. A. et al. Reactivity of a stable copper-dioxygen complex. Chem. Commun. 53, 10306–10309 (2017).
Peterson, R. L. et al. Cupric superoxo-mediated intermolecular C–H activation chemistry. J. Am. Chem. Soc. 133, 1702–1705 (2011).
Tomson, N. C. et al. Re-evaluating the Cu K pre-edge XAS transition in complexes with covalent metal–ligand interactions. Chem. Sci. 6, 2474–2487 (2015).
Hedegård, E. D. & Ryde, U. Targeting the reactive intermediate in polysaccharide monooxygenases. J. Biol. Inorg. Chem. 22, 1029–1037 (2017).
Wang, B. et al. QM/MM Studies into the H2O2-dependent activity of lytic polysaccharide monooxygenases: evidence for the formation of a caged hydroxyl radical intermediate. ACS Catalysis 8, 1346–1351 (2018).
Neisen, B. D. et al. Formally copper(iii)–alkylperoxo complexes as models of possible intermediates in monooxygenase enzymes. J. Am. Chem. Soc. 139, 10220–10223 (2017).
Yamaguchi, S. & Masuda, H. Basic approach to development of environment-friendly oxidation catalyst materials. Mononuclear hydroperoxo copper(ii) complexes. Sci. Technol. Adv. Mater. 6, 34–47 (2005).
Kitajima, N. et al. Synthesis, molecular structure, and reactivity of (alkylperoxo)copper(ii) complex. J. Am. Chem. Soc. 115, 7872–7873 (1993).
Peterson, R. L. et al. Stepwise protonation and electron-transfer reduction of a primary copper–dioxygen adduct. J. Am. Chem. Soc. 135, 16454–16467 (2013).
Trammell, R. et al. Decoding the mechanism of intramolecular Cu-directed hydroxylation of sp 3 C–H bonds. J. Org. Chem. 82, 7887–7904 (2017).
Maiti, D. et al. Reactions of a copper(ii) superoxo complex lead to C–H and O–H substrate oxygenation: modeling copper-monooxygenase C–H hydroxylation. Angew. Chem. Int. Ed. 47, 82–85 (2008).
Tano, T. et al. Reactivity of copper(ii)-alkylperoxo complexes. Dalton Trans. 40, 10326–10336 (2011).
Bertini, L. et al. Catalytic mechanism of fungal lytic polysaccharide monooxygenases investigated by first-principles calculations. Inorg. Chem. 57, 86–97 (2018).
Osborne, R. L. & Klinman, J. P. in Copper-Oxygen Chemistry (eds Karlin, K. D., Itoh, S.) Ch. 1, 1–22 (John Wiley & Sons, 2011).
Dietl, N. et al. Diatomic [CuO]+ and its role in the spin-selective hydrogen- and oxygen-atom transfers in the thermal activation of methane. Angew. Chem. Int. Ed. 50, 4966–4969 (2011).
Schröder, D., Holthausen, M. C. & Schwarz, H. Radical-like activation of alkanes by the ligated copper oxide cation (Phenanthroline)CuO+. J. Phys. Chem. B 108, 14407–14416 (2004).
Yoshizawa, K., Kihara, N., Kamachi, T. & Shiota, Y. Catalytic mechanism of dopamine β-monooxygenase mediated by Cu(iii)−oxo. Inorg. Chem. 45, 3034–3041 (2006).
Dietl, N., Schlangen, M. & Schwarz, H. Thermal hydrogen-atom transfer from methane: the role of radicals and spin states in oxo-cluster chemistry. Angew. Chem. Int. Ed. 51, 5544–5555 (2012).
Donoghue, P. J. et al. Rapid C–H bond activation by a monocopper(iii)–hydroxide complex. J. Am. Chem. Soc. 133, 17602–17605 (2011).
Dhar, D. & Tolman, W. B. Hydrogen atom abstraction from hydrocarbons by a copper(iii)–hydroxide complex. J. Am. Chem. Soc. 137, 1322–1329 (2015).
Dhar, D. et al. Perturbing the copper(iii)–hydroxide unit through ligand structural variation. J. Am. Chem. Soc. 138, 356–368 (2016).
Dhar, D. et al. Reactivity of the copper(iii)–hydroxide unit with phenols. Chem. Sci. 8, 1075–1085 (2017).
Spaeth, A. D. et al. Determination of the Cu(iii)–OH bond distance by resonance raman spectroscopy using a normalized version of badger’s rule. J. Am. Chem. Soc. 139, 4477–4485 (2017).
Donoghue, P. J. et al. An anionic, tetragonal copper(ii) superoxide complex. J. Am. Chem. Soc. 132, 15869–15871 (2010).
Pirovano, P. et al. Nucleophilic reactivity of a copper(ii)–superoxide complex. Angew. Chem. Int. Ed. 126, 6056–6060 (2014).
Acknowledgements
W.B.T. thanks the US National Institute of Health (grant R37GM47365) for financial support. L.C., G.J.D. and P.H.W. gratefully acknowledge the support of the UK’s Biotechnology and Biological Sciences Research Council (BBSRC) through grants BB/L001926/1 and BB/L021633/1. G.J.D. is a Royal Society Ken Murray Research Professor.
Author information
Authors and Affiliations
Contributions
W.B.T. and P.H.W. wrote the manuscript, G.J.D. analysed LPMO and pMMO. protein structures and contributed to the writing of the manuscript. L.C. prepared metrical data for structure analysis and contributed to the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–3
Supplementary Data Set 1
Data from Supplementary Table 1: Collective metrics for copper histidine brace active site in known LPMO structures
Rights and permissions
About this article
Cite this article
Ciano, L., Davies, G.J., Tolman, W.B. et al. Bracing copper for the catalytic oxidation of C–H bonds. Nat Catal 1, 571–577 (2018). https://doi.org/10.1038/s41929-018-0110-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-018-0110-9
This article is cited by
-
Expanding the catalytic landscape of metalloenzymes with lytic polysaccharide monooxygenases
Nature Reviews Chemistry (2024)
-
Structural perturbations of substrate binding and oxidation state changes in a lytic polysaccharide monooxygenase
JBIC Journal of Biological Inorganic Chemistry (2022)
-
Recent Computational Insights into the Oxygen Activation by Copper-Dependent Metalloenzymes
Topics in Catalysis (2022)
-
Deciphering the oxygen activation mechanism at the CuC site of particulate methane monooxygenase
Nature Catalysis (2021)
-
Current state and future perspectives of engineered and artificial peroxygenases for the oxyfunctionalization of organic molecules
Nature Catalysis (2020)