Abstract
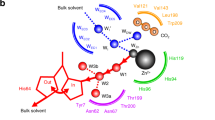
Non-canonical amino acid ligands are useful for fine-tuning the catalytic properties of metalloenzymes. Here, we show that recombinant replacement of the histidine ligand proximal to haem in myoglobin with Nδ-methylhistidine enhances the protein’s promiscuous carbene-transfer chemistry, enabling efficient styrene cyclopropanation in the absence of reductant, even under aerobic conditions. The increased electrophilicity of the modified Fe(iii) centre, combined with subtle structural adjustments at the active site, allows direct attack of ethyl diazoacetate to produce a reactive carbenoid adduct, which has an unusual bridging Fe(iii)–C–N(pyrrole) configuration as shown by X-ray crystallography. Quantum chemical calculations suggest that the bridged complex equilibrates with the more reactive end-on isomer, ensuring efficient cyclopropanation. These findings underscore the potential of non-canonical ligands for extending the capabilities of metalloenzymes by opening up new reaction pathways and facilitating the characterization of reactive species that would not otherwise accumulate.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Schwizer, F. et al. Artificial metalloenzymes: reaction scope and optimization strategies. Chem. Rev. 118, 142–231 (2018).
Poulos, T. L. Heme enzyme structure and function. Chem. Rev. 114, 3919–3962 (2014).
Wang, J. Z., Peck, N. E., Renata, H. & Arnold, F. H. Cytochrome P450-catalyzed insertion of carbenoids into N–H bonds. Chem. Sci. 5, 598–601 (2014).
Sreenilayam, G. & Fasan, R. Myoglobin-catalyzed intermolecular carbene N–H insertion with arylamine substrates. Chem. Commun. 51, 1532–1534 (2015).
Tyagi, V., Bonn, R. B. & Fasan, R. Intermolecular carbene S–H insertion catalysed by engineered myoglobin-based catalysts. Chem. Sci. 6, 2488–2494 (2015).
Coelho, P. S., Brustad, E. M., Kannan, A. & Arnold, F. H. Olefin cyclopropanation via carbene transfer catalyzed by engineered cytochrome P450 enzymes. Science 339, 307–310 (2013).
Bordeaux, M., Tyagi, V. & Fasan, R. Highly diastereoselective and enantioselective olefin cyclopropanation using engineered myoglobin-based catalysts. Angew. Chem. Int. Ed. 54, 1744–1748 (2015).
Coelho, P. S. et al. A serine-substituted P450 catalyzes highly efficient carbene transfer to olefins in vivo. Nat. Chem. Biol. 9, 485–487 (2013).
Key, H. M., Dydio, P., Clark, D. S. & Hartwig, J. F. Abiological catalysis by artificial haem proteins containing noble metals in place of iron. Nature 534, 534–537 (2016).
Sreenilayam, G., Moore, E. J., Steck, V. & Fasan, R. Metal substitution modulates the reactivity and extends the reaction scope of myoglobin carbene transfer catalysts. Adv. Synth. Catal. 359, 2076–2089 (2017).
Sreenilayam, G., Moore, E. J., Steck, V. & Fasan, R. Stereoselective olefin cyclopropanation under aerobic conditions with an artificial enzyme incorporating an iron-chlorin e6 cofactor. ACS Catal. 7, 7629–7633 (2017).
Oohora, K. et al. Catalytic cyclopropanation by myoglobin reconstituted with iron porphycene: acceleration of catalysis due to rapid formation of the carbene species. J. Am. Chem. Soc. 139, 17265–17268 (2017).
Liu, C. C. & Schultz, P. G. Adding new chemistries to the genetic code. Annu. Rev. Biochem. 79, 413–444 (2010).
Lewis, J. C. Metallopeptide catalysts and artificial metalloenzymes containing unnatural amino acids. Curr. Opin. Chem. Biol. 25, 27–35 (2015).
Pott, M. et al. A non-canonical proximal heme ligand affords an efficient peroxidase in a globin fold. J. Am. Chem. Soc. 140, 1535–1543 (2018).
Green, A. P., Hayashi, T., Mittl, P. R. E. & Hilvert, D. A chemically programmed proximal ligand enhances the catalytic properties of a heme enzyme. J. Am. Chem. Soc. 138, 11344–11352 (2016).
Bhagi-Damodaran, A., Petrik, I. D., Marshall, N. M., Robinson, H. & Lu, Y. Systematic tuning of heme redox potentials and its effects on O2 reduction rates in a designed oxidase in myoglobin. J. Am. Chem. Soc. 136, 11882–11885 (2014).
Xiao, H. et al. Genetic incorporation of histidine derivatives using an engineered pyrrolysyl-tRNA synthetase. ACS Chem. Biol. 9, 1092–1096 (2014).
Springer, B. A. & Sligar, S. G. High-level expression of sperm whale myoglobin in Escherichia coli. Proc. Natl Acad. Sci. USA 84, 8961–8965 (1987).
Gurd, F. R., Falk, K. E., Malmström, B. G. & Vänngård, T. A magnetic resonance study of sperm whale ferrimyoglobin and its complex with 1 cupric ion. J. Biol. Chem. 242, 5724–5730 (1967).
Mbuvi, H. M. & Woo, L. K. Catalytic C–H insertions using iron(III) porphyrin complexes. Organometallics 27, 637–645 (2008).
Baumann, L. K., Mbuvi, H. M., Du, G. & Woo, L. K. Iron porphyrin catalyzed N–H insertion reactions with ethyl diazoacetate. Organometallics 26, 3995–4002 (2007).
Li, Y., Huang, J. S., Zhou, Z. Y., Che, C. M. & You, X. Z. Remarkably stable iron porphyrins bearing nonheteroatom-stabilized carbene or (alkoxycarbonyl)carbenes: isolation, X-ray crystal structures, and carbon atom transfer reactions with hydrocarbons. J. Am. Chem. Soc. 124, 13185–13193 (2002).
Liu, Y. et al. Electronic configuration and ligand nature of five-coordinate iron porphyrin carbene complexes: an experimental study. J. Am. Chem. Soc. 139, 5023–5026 (2017).
Smith, D. A., Heeg, M. J., Heineman, W. R. & Elder, R. C. Direct determination of Fe–C bond lengths in iron(II) and iron(III) cyanide solutions using EXAFS spectroelectrochemistry. J. Am. Chem. Soc. 106, 3053–3054 (1984).
Simonneaux, G. & Le Maux, P. Carbene complexes of heme proteins and iron porphyrin models. Top. Organomet. Chem. 17, 83–122 (2006).
Latos-Grazynski, L., Cheng, R. J., La Mar, G. N. & Balch, A. L. Reversible migration of an axial carbene ligand into an iron–nitrogen bond of a porphyrin. Implications for high oxidation states of heme enzymes and heme catabolism. J. Am. Chem. Soc. 103, 4270–4272 (1981).
Chevrier, B., Weiss, R., Lange, M., Chottard, J. C. & Mansuy, D. An iron(III)–porphyrin complex with a vinylidene group inserted into an iron–nitrogen bond: relevance to the structure of the active oxygen complex of catalase. J. Am. Chem. Soc. 103, 2899–2901 (1981).
Yeung, N. et al. Rational design of a structural and functional nitric oxide reductase. Nature 462, 1079–1082 (2009).
Jentzen, W., Ma, J.-G. & Shelnutt, J. A. Conservation of the conformation of the porphyrin macrocycle in hemoproteins. Biophys. J. 74, 753–763 (1998).
Renata, H. et al. Identification of mechanism-based inactivation in P450-catalyzed cyclopropanation facilitates engineering of improved enzymes. J. Am. Chem. Soc. 138, 12527–12533 (2016).
Callot, H. J. & Tschamber, T. Rearrangement of N-substituted porphyrins. Preparation and structure of homoporphyrins. J. Am. Chem. Soc. 97, 6175–6178 (1975).
Khade, R. L. & Zhang, Y. Catalytic and biocatalytic iron porphyrin carbene formation: effects of binding mode, carbene substituent, porphyrin substituent, and protein axial ligand. J. Am. Chem. Soc. 137, 7560–7563 (2015).
Dzik, W. I., Xu, X., Zhang, X. P., Reek, J. N. H. & de Bruin, B. ‘Carbene radicals’ in CoII(por)-catalyzed olefin cyclopropanation. J. Am. Chem. Soc. 132, 10891–10902 (2010).
Carminati, D. M. et al. Designing ‘totem’ C2-symmetrical iron porphyrin catalysts for stereoselective cyclopropanations. Chem. Eur. J. 22, 13599–13612 (2016).
Kan, S. B. J., Lewis, R. D., Chen, K. & Arnold, F. H. Directed evolution of cytochrome c for carbon–silicon bond formation: bringing silicon to life. Science 354, 1048–1051 (2016).
Kan, S. B. J., Huang, X., Gumulya, Y., Chen, K. & Arnold, F. H. Genetically programmed chiral organoborane synthesis. Nature 552, 132–136 (2017).
Rioz-Martínez, A., Oelerich, J., Ségaud, N. & Roelfes, G. DNA-accelerated catalysis of carbene-transfer reactions by a DNA/cationic iron porphyrin hybrid. Angew. Chem. Int. Ed. 55, 14136–14140 (2016).
Nicolas, I., Maux, P., Le & Simonneaux, G. Synthesis of chiral water-soluble metalloporphyrins (Fe, Ru,): new catalysts for asymmetric carbene transfer in water. Tetrahedron Lett. 49, 5793–5795 (2008).
Intrieri, D. et al. Highly diastereoselective cyclopropanation of α-methylstyrene catalysed by a C2-symmetrical chiral iron porphyrin complex. Chem. Commun. 50, 1811–1813 (2014).
Zeldin, O. B., Gerstel, M. & Garman, E. F. RADDOSE-3D: time- and space-resolved modelling of dose in macromolecular crystallography. J. Appl. Crystallogr. 46, 1225–1230 (2013).
Kabsch, W. XDS. Acta Crystallogr. D 66, 125–132 (2010).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Vojtěchovský, J., Chu, K., Berendzen, J., Sweet, R. M. & Schlichting, I. Crystal structures of myoglobin–ligand complexes at near-atomic resolution. Biophys. J. 77, 2153–2174 (1999).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Afonine, P. V. et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D 68, 352–367 (2012).
Terwilliger, T. C. et al. Iterative-build OMIT maps: map improvement by iterative model building and refinement without model bias. Acta Crystallogr. D 64, 515–524 (2008).
Liebschner, D. et al. Polder maps: improving OMIT maps by excluding bulk solvent. Acta Crystallogr. D 73, 148–157 (2017).
Berry, E. A. & Trumpower, B. L. Simultaneous determination of hemes a, b, and c from pyridine hemochrome spectra. Anal. Biochem. 161, 1–15 (1987).
Williams, J. N. A method for the simultaneous quantitative estimation of cytochromes a, b, c1, and c in mitochondria. Arch. Biochem. Biophys. 107, 537–543 (1964).
Efimov, I. et al. A simple method for the determination of reduction potentials in heme proteins. FEBS Lett. 588, 701–704 (2014).
Stoll, S. & Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 178, 42–55 (2006).
Frisch, M. J. E. A. et al. Gaussian 09, revision D.01 (Gaussian, Inc., 2009).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 8, 1057–1065 (2006).
Adamo, C. & Barone, V. Toward reliable density functional methods without adjustable parameters: the PBE0 model. J. Chem. Phys. 110, 6158–6170 (1999).
Reiher, M., Salomon, O. & Hess, B. A. Reparameterization of hybrid functionals based on energy differences of states of different multiplicity. Theor. Chem. Acc. 107, 48–55 (2001).
Peng, C. & Bernhard Schlegel, H. Combining synchronous transit and quasi-Newton methods to find transition states. Isr. J. Chem. 33, 449–454 (1993).
Fukui, K. The path of chemical reactions—the IRC approach. Acc. Chem. Res. 14, 363–368 (1981).
Acknowledgements
We thank the Swiss Light Source team at the Paul Scherrer Institute for technical assistance, the Service for Mass Spectrometry (ETH Zürich) for carrying out the HRMS measurements, and T. Sandmeier for assistance with the SFC measurements. We are grateful to E. Carreira and P. Chen for access to the SFC and gas chromatography instruments, and A. Mezzetti and P. Pregosin for helpful discussions. This work was generously supported by ETH Zürich and the Swiss National Science Foundation.
Author information
Authors and Affiliations
Contributions
T.H., M.T. and D.H. designed the research. T.H., M.T. and T.M. carried out the experiments. U.K. provided guidance for the X-ray analysis and validated the crystal structures. J.P. and M.R. designed the computational methodology. J.P. carried out the calculations, and J.P. and M.R. then analysed the data obtained. J.S. and D.K. carried out the EPR measurements under the guidance of G.J. All authors contributed to writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hayashi, T., Tinzl, M., Mori, T. et al. Capture and characterization of a reactive haem–carbenoid complex in an artificial metalloenzyme. Nat Catal 1, 578–584 (2018). https://doi.org/10.1038/s41929-018-0105-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-018-0105-6
This article is cited by
-
Mechanistic and structural characterization of an iridium-containing cytochrome reveals kinetically relevant cofactor dynamics
Nature Catalysis (2023)
-
Mechanistic manifold in a hemoprotein-catalyzed cyclopropanation reaction with diazoketone
Nature Communications (2023)
-
The road to fully programmable protein catalysis
Nature (2022)
-
Recent advancements in enzyme engineering via site-specific incorporation of unnatural amino acids
World Journal of Microbiology and Biotechnology (2021)
-
Expanding the enzyme universe with genetically encoded unnatural amino acids
Nature Catalysis (2020)