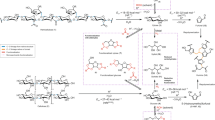
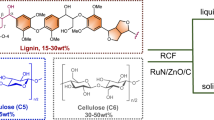
Abstract
Lignocellulose, the main component of agricultural and forestry waste, harbours tremendous potential as a renewable starting material for future biorefinery practices. However, this potential remains largely unexploited due to the lack of strategies that derive substantial value from its main constituents. Here, we present a catalytic strategy that is able to transform lignocellulose to a range of attractive products. At the centre of our approach is the flexible use of a non-precious metal catalyst in two distinct stages of a lignocellulose conversion process that enables integrated catalyst recycling through full conversion of all process residues. From the lignin, pharmaceutical and polymer building blocks are obtained. Notably, among these pathways are systematic chemo-catalytic methodologies to yield amines from lignin. The (hemi)cellulose-derived aliphatic alcohols are transformed to alkanes, achieving excellent total carbon utilization. This work will inspire the development of fully sustainable and economically viable biorefineries.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Tuck, C. O., Perez, E., Horváth, I. T., Sheldon, R. A. & Poliakoff, M. Valorization of biomass: deriving more value from waste. Science 337, 695–699 (2012).
Christopher, L., Clark, J. H. & Kraus, G. A. Integrated Forest Biorefineries: Challenges and Opportunities (Royal Society of Chemistry, London, 2012).
Zaheer, M. & Kempe, R. Catalytic hydrogenolysis of aryl ethers: a key step in lignin valorization to valuable chemicals. ACS Catal. 5, 1675–1684 (2015).
Galkin, M. V. & Samec, J. S. M. Lignin valorization through catalytic lignocellulose fractionation: a fundamental platform for the future biorefinery. ChemSusChem 9, 1544–1558 (2016).
Deuss, P. J. et al. Phenolic acetals from lignins of varying compositions via iron(III) triflate catalysed depolymerisation. Green Chem. 19, 2774–2782 (2017).
Zakzeski, J., Bruijnincx, P. C. A., Jongerius, A. L. & Weckhuysen, B. M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 110, 3552–3599 (2010).
Xu, C., Arneil, R., Arancon, D., Labidi, J. & Luque, R. Lignin depolymerisation strategies: towards valuable chemicals and fuels. Chem. Soc. Rev. 43, 7485–7500 (2014).
Deuss, P. J. & Barta, K. From models to lignin: transition metal catalysis for selective bond cleavage reactions. Coord. Chem. Rev. 306, 510–532 (2016).
Hanson, S. K. & Baker, R. T. Knocking on wood: base metal complexes as catalysts for selective oxidation of lignin models and extracts. Acc. Chem. Res. 48, 2037–2048 (2015).
Rahimi, A., Ulbrich, A., Coon, J. J. & Stahl, S. S. Formic-acid-induced depolymerization of oxidized lignin to aromatics. Nature 515, 249–252 (2014).
Shuai, L. et al. Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization. Science 354, 329–333 (2016).
Lancefield, C. S., Ojo, O. S., Tran, F. & Westwood, N. J. Isolation of functionalized phenolic monomers through selective oxidation and C-O bond cleavage of the β-O-4 linkages in lignin. Angew. Chem. Int. Ed. 54, 258–262 (2015).
Linger, J. G. et al. Lignin valorization through integrated biological funneling and chemical catalysis. Proc. Natl Acad. Sci. USA 111, 12013–12018 (2014).
Feghali, E., Carrot, G., Thuéry, P., Genre, C. & Cantat, T. Convergent reductive depolymerization of wood lignin to isolated phenol derivatives by metal-free catalytic hydrosilylation. Energy Environ. Sci. 8, 2734–2743 (2015).
Ferrini, P. & Rinaldi, R. Catalytic biorefining of plant biomass to non-pyrolytic lignin bio-oil and carbohydrates through hydrogen transfer reactions. Angew. Chem. Int. Ed. 53, 8634–8639 (2014).
Van den Bosch, S. et al. Reductive lignocellulose fractionation into soluble lignin-derived phenolic monomers and dimers and processable carbohydrate pulps. Energy Environ. Sci. 8, 1748–1763 (2015).
Parsell, T. et al. A synergistic biorefinery based on catalytic conversion of lignin prior to cellulose starting from lignocellulosic biomass. Green Chem. 17, 1492–1499 (2015).
Barta, K. & Ford, P. C. Catalytic conversion of nonfood woody biomass solids to organic liquids. Acc. Chem. Res. 47, 1503–1512 (2014).
Bähn, S. et al. The catalytic amination of alcohols. ChemCatChem 3, 1853–1864 (2011).
Imm, S., Bähn, S., Neubert, L., Neumann, H. & Beller, M. An efficient and general synthesis of primary amines by ruthenium-catalyzed amination of secondary alcohols with ammonia. Angew. Chem. Int. Ed. 49, 8126–8129 (2010).
Sutton, A. D. et al. The hydrodeoxygenation of bioderived furans into alkanes. Nat. Chem. 5, 428–432 (2013).
Nichols, J. M., Bishop, L. M., Bergman, R. G. & Ellman, J. A. Catalytic C–O bond cleavage of 2-aryloxy-1-arylethanols and its application to the depolymerization of lignin-related polymers. J. Am. Chem. Soc. 132, 12554–12555 (2010).
Deuss, P. J. et al. Aromatic monomers by in situ conversion of reactive intermediates in the acid-catalyzed depolymerization of lignin. J. Am. Chem. Soc. 137, 7456–7467 (2015).
Anbarasan, P. et al. Integration of chemical catalysis with extractive fermentation to produce fuels. Nature 491, 235–239 (2012).
Sreekumar, S. et al. Production of an acetone-butanol-ethanol mixture from Clostridium acetobutylicum and its conversion to high-value biofuels. Nat. Protoc. 10, 528–537 (2015).
Yang, J. et al. Synthesis of diesel and jet fuel range alkanes with furfural and ketones from lignocellulose under solvent free conditions. Green. Chem. 16, 4879–4884 (2014).
Li, G. et al. Synthesis of diesel or jet fuel range cycloalkanes with 2-methylfuran and cyclopentanone from lignocellulose. Energy Fuels 28, 5112–5118 (2014).
Sun, Z. et al. Efficient catalytic conversion of ethanol to 1-butanol via the Guerbet reaction over copper- and nickel-doped porous metal oxides. ACS Sustain. Chem. Eng. 5, 1738–1746 (2017).
Mycroft, Z., Gomis, M., Mines, P., Law, P. & Bugg, T. D. H. Biocatalytic conversion of lignin to aromatic dicarboxylic acids in Rhodococcus jostii RHA1 by re-routing aromatic degradation pathways. Green Chem. 17, 4974–4979 (2015).
Shimizu, K. I., Kon, K., Onodera, W., Yamazaki, H. & Kondo, J. N. Heterogeneous Ni catalyst for direct synthesis of primary amines from alcohols and ammonia. ACS Catal. 3, 112–117 (2013).
Gunanathan, C. & Milstein, D. Selective synthesis of primary amines directly from alcohols and ammonia. Angew. Chem. Int. Ed. 47, 8661–8664 (2008).
Jagadeesh, R. V., Junge, H. & Beller, M. Green synthesis of nitriles using non-noble metal oxides-based nanocatalysts. Nat. Commun. 5, 4123 (2014).
Gooβen, L. J. & Rodríguez, N. A mild and efficient protocol for the conversion of carboxylic acids to olefins by a catalytic decarbonylative elimination reaction. Chem. Commun. 0, 724–725 (2004).
Lavoie, C. M. et al. Challenging nickel-catalysed amine arylations enabled by tailored ancillary ligand design. Nat. Commun. 7, 11073 (2016).
Tobisu, M. & Chatani, N. Cross-couplings using aryl ethers via C–O bond activation enabled by nickel catalysts. Acc. Chem. Res. 48, 1717–1726 (2015).
Álvarez-Bercedo, P. & Martin, R. Ni-catalyzed reduction of inert C–O bonds: A new strategy for using aryl ethers as easily removable directing groups. J. Am. Chem. Soc. 132, 17352–17353 (2010).
St Amant, A. H., Frazier, C. P., Newmeyer, B., Fruehauf, K. R. & Read de Alaniz, J. Direct synthesis of anilines and nitrosobenzenes from phenols. Org. Biomol. Chem. 14, 5520–5524 (2016).
Plourde, G. L. Studies towards the diastereoselective spiroannulation of phenolic derivatives. Tetrahedron Lett. 43, 3597–3599 (2002).
Hamid, M. H. S. A. et al. Ruthenium-catalyzed N-alkylation of amines and sulfonamides using borrowing hydrogen methodology. J. Am. Chem. Soc. 131, 1766–1774 (2009).
Zhao, S. & Abu-Omar, M. M. Biobased epoxy nanocomposites derived from lignin-based monomers. Biomacromolecules 16, 2025–2031 (2015).
Koelewijn, S.-F. et al. Sustainable bisphenols from renewable softwood lignin feedstock for polycarbonates and cyanate ester resins. Green Chem. 19, 2561–2570 (2017).
Llevot, A., Grau, E., Carlotti, S., Grelier, S. & Cramail, H. From lignin-derived aromatic compounds to novel biobased polymers. Macromol. Rapid Commun. 37, 9–28 (2016).
Upton, B. M. & Kasko, A. M. Strategies for the conversion of lignin to high-value polymeric materials: review and perspective. Chem. Rev. 116, 2275–2306 (2016).
Takeshima, H., Satoh, K. & Kamigaito, M. Bio-based functional styrene monomers derived from naturally occurring ferulic acid for poly(vinylcatechol) and poly(vinylguaiacol) via controlled radical polymerization. Macromolecules 50, 4206–4216 (2017).
Miura, Y., Hoshino, Y. & Seto, H. Glycopolymer nanobiotechnology. Chem. Rev. 116, 1673–1692 (2016).
Wilker, J. J. Biomaterials: redox and adhesion on the rocks. Nat. Chem. Biol. 7, 579–580 (2011).
Hutchinson, S. A., Luetjens, H. & Scammells, P. J. A new synthesis of the benzofuran adenosine antagonist XH-14. Bioorg. Med. Chem. Lett. 7, 3081–3084 (1997).
Schlepphorst, C., Maji, B. & Glorius, F. Ruthenium-NHC catalyzed α-alkylation of methylene ketones provides branched products through borrowing hydrogen strategy. ACS Catal. 6, 4184–4188 (2016).
Ackermann, L. & Althammer, A. Domino N-H/C-H bond activation: palladium-catalyzed synthesis of annulated heterocycles using dichloro(hetero)arenes. Angew. Chem. Int. Ed. 46, 1627–1629 (2007).
Lei, M., Gan, X., Zhao, K., Yu, Q. & Hu, L. Synthesis and cytotoxicity evaluation of 4-amino-4-dehydroxylarctigenin derivatives in glucose-starved A549 tumor cells. Bioorg. Med. Chem. Lett. 25, 435–437 (2015).
Acknowledgements
K.B. is grateful for financial support from the European Research Council, ERC Starting Grant 2015 (CatASus) 638076. This work is part of the research programme Talent Scheme (Vidi) with project number 723.015.005 (K.B.), which is partly financed by the Netherlands Organisation for Scientific Research (NWO). Z.S. is grateful for financial support from the China Scholarship Council (grant number 201406060027). B.F. is grateful for the financial support from the Hungarian Ministry of Human Capacities (NTP-NFTÖ-17-B-0593).
Author information
Authors and Affiliations
Contributions
K.B. conceived the idea, supervised the research and wrote the manuscript. Z.S. designed the LignoFlex process and performed all related chemical reactions. Z.S. also performed reactions related to Stage 2 and synthesized compounds 3G, 4 and 6. G.B. and A.A. contributed equally to this research and designed pathways for the functionalization of 1G and synthesized compounds 5, 7, 7S, 8, 9a–e, 10, 11, 12a–c, 13, 14 and 15. M.C.A.S. performed catalyst characterization. P.J.D. measured and analysed the 2D-HSQC NMR data and was involved in figure preparation. B.F. contributed to the catalytic conversion of alcohol mixture to alkanes, and designed synthetic pathways to obtain compound 6Cy. All of the authors commented on the manuscript during its preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–93, Supplementary Tables 1–21, Supplementary Notes 1–14, Supplementary Methods, Supplementary References.
Rights and permissions
About this article
Cite this article
Sun, Z., Bottari, G., Afanasenko, A. et al. Complete lignocellulose conversion with integrated catalyst recycling yielding valuable aromatics and fuels. Nat Catal 1, 82–92 (2018). https://doi.org/10.1038/s41929-017-0007-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-017-0007-z
This article is cited by
-
Enhancement of cellulolytic enzyme production from intrageneric protoplast fusion of Aspergillus species and evaluating the hydrolysate scavenging activity
Microbial Cell Factories (2024)
-
Carbon–carbon bond cleavage for a lignin refinery
Nature Chemical Engineering (2024)
-
Sustainable production of dopamine hydrochloride from softwood lignin
Nature Communications (2023)
-
Multilamellar spherical micelles of alkali lignin: dissipative particle dynamics simulations
Journal of Molecular Modeling (2023)
-
Transforming Lignin Biomass to Value: Interplay Between Ligninolytic Enzymes and Lignocellulose Depolymerization
BioEnergy Research (2023)